
Leopold Ružička was a Croatian-Swiss scientist and joint winner of the 1939 Nobel Prize in Chemistry "for his work on polymethylenes and higher terpenes" "including the first chemical synthesis of male sex hormones." He worked most of his life in Switzerland, and received eight doctorates honoris causa in science, medicine, and law; seven prizes and medals; and twenty-four honorary memberships in chemical, biochemical, and other scientific societies.

Acetoin, also known as 3-hydroxybutanone or acetyl methyl carbinol, is an organic compound with the formula CH3CH(OH)C(O)CH3. It is a colorless liquid with a pleasant, buttery odor. It is chiral. The form produced by bacteria is (R)-acetoin.

Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (an amide of phosphoric acid) with the formula [(CH3)2N]3PO. This colorless liquid is used as a solvent in organic synthesis.

Calone or methylbenzodioxepinone, trade-named Calone 1951, is an organic compound with the formula CH3C6H3(OCH2)2CO. A white solid, it is a derivative of 4-methylcatechol. In the fragrance industry it is known as "watermelon ketone".

2-Aminothiazole is a heterocyclic amine featuring a thiazole core. It can also be considered a cyclic isothiourea. It possesses an odor similar to pyridine and is soluble in water, alcohols and diethyl ether. 2-Aminothiazole itself is mainly of academic interest, with few exceptions. It is a precursor to a sulfathiazole. 2-Aminothiazole can be used as a thyroid inhibitor in the treatment of hyperthyroidism.

Albert Jakob Eschenmoser (5 August 1925 – 14 July 2023) was a Swiss organic chemist, best known for his work on the synthesis of complex heterocyclic natural compounds, most notably vitamin B12. In addition to his significant contributions to the field of organic synthesis, Eschenmoser pioneered work in the Origins of Life (OoL) field with work on the synthetic pathways of artificial nucleic acids. Before retiring in 2009, Eschenmoser held tenured teaching positions at the ETH Zurich and The Skaggs Institute for Chemical Biology at The Scripps Research Institute in La Jolla, California as well as visiting professorships at the University of Chicago, Cambridge University, and Harvard.
2-Methylundecanal is an organic compound that is found naturally in kumquat peel oil. This compound smells herbaceous, orange, and ambergris-like. At high dilution it has a flavor similar to honey and nuts. It is a colorless or pale yellow liquid that is soluble in organic solvents such as ether and ethanol. It is used as a fragrance component in soaps, detergents, and perfumes.
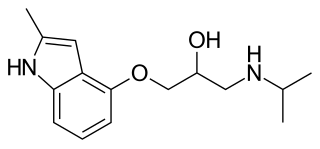
Mepindolol (Betagon) is a non-selective beta blocker. It is used to treat glaucoma.
Synthetic musks are a class of synthetic aroma compounds to emulate the scent of deer musk and other animal musks. Synthetic musks have a clean, smooth and sweet scent lacking the fecal notes of animal musks. They are used as flavorings and fixatives in cosmetics, detergents, perfumes and foods, supplying the base note of many perfume formulas. Most musk fragrance used in perfumery today is synthetic.

Roman Kaiser is a Swiss fragrance chemist. Since 1968 he has been working at Givaudan, the world's largest company in the flavour and fragrance industry, where he analyzes and reconstitutes natural scents for use in perfumery using the headspace technology which he pioneered and which as a new concept made significant impact on the analysis of natural products.

Paul Parquet (1856–1916) was a French perfumer and joint owner of Houbigant. Called the "greatest perfumer of his time" by Ernest Beaux, he is widely regarded as the founder of modern perfumery for having pioneered the use of synthetics in works such as Fougère Royale. His bestselling perfume, Le Parfum Idéal, was described by Robert Bienaimé as a “masterpiece of fragrant equilibrium, harmonious and of good taste as shall never be surpassed”.
A captive odorant, or short captive, is an odorant or aroma chemical retained by the originating manufacturer for exclusive use in their own perfumes to protect them from imitation.
The total synthesis of the complex biomolecule vitamin B12 was accomplished in two different approaches by the collaborating research groups of Robert Burns Woodward at Harvard and Albert Eschenmoser at ETH in 1972. The accomplishment required the effort of no less than 91 postdoctoral researchers (Harvard: 77, ETH: 14), and 12 Ph.D. students (at ETH) from 19 different nations over a period of almost 12 years. The synthesis project induced and involved a major change of paradigm in the field of natural product synthesis.
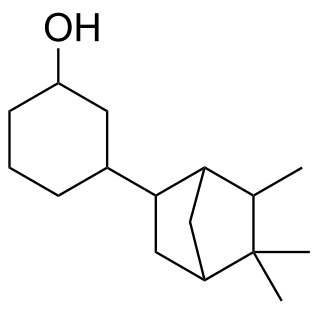
Isobornyl cyclohexanol is an organic compound used primarily as a fragrance because of its aroma which is similar to sandalwood oil. Its chemical structure is closely related to that of both α-santalol and β-santalol, which are the primary constituents of sandalwood oil.
Günther Ohloff was a prominent German fragrance chemist.

Galaxolide is a synthetic musk with a clean sweet musky floral woody odor used in fragrances. It is one of the musk components that perfume and cologne manufacturers use to add a musk odor to their products. Galaxolide was first synthesized in 1956, and used in the late 1960s in some fabric softeners and detergents. High concentrations were also incorporated in fine fragrances.

Pomarose is a high-impact captive odorant patented by Givaudan. It is a double-unsaturated ketone that does not occur in nature. Pomarose has a powerful fruity rose odor with nuances of apples, plums and raisins, which is almost entirely due to the (2E,5Z)-stereoisomer, while its (2E,5E)-isomer is barely detectable for most people. Catalyzed by traces of acids, both isomers equilibrate however quickly upon standing in glass containers.
Ketonic decarboxylation is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid to a symmetric ketone by the application of heat. It can be thought of as a decarboxylative Claisen condensation of two identical molecules. Water and carbon dioxide are byproducts:
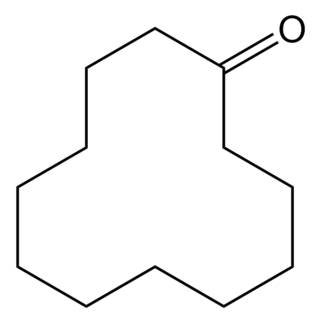
Cyclododecanone is an organic compound with the formula (CH2)11CO. It is a cyclic ketone that exists as a white solid at room temperature. Like its smaller analogs but unlike the larger ones, it has a camphor-like odor.

1,2,4,5-Cyclohexanetetrol (also named cyclohexane-1,2,4,5-tetrol, 1,2,4,5-tetrahydroxycyclohexane, or para-cyclohexanetetrol) is an organic compound whose molecule can be described as a cyclohexane with four hydroxyl (OH) groups substituted for hydrogen atoms on two non-adjacent pairs of adjacent carbon atoms. Its formula can be written C
6H
12O
4, C
6H
8(OH)
4, or [–(CH(OH)–)2–CH
2–]2.














