Related Research Articles
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract.

In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ligare, which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure.

The Journal of Medicinal Chemistry is a biweekly peer-reviewed medical journal covering research in medicinal chemistry. It is published by the American Chemical Society. It was established in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry and obtained its current name in 1963. Philip S. Portoghese served as editor-in-chief from 1972 to 2011. In 2012, Gunda Georg and Shaomeng Wang succeeded Portoghese. In 2021, Craig W. Lindsley became editor-in-chief. According to the Journal Citation Reports, the journal has a 2021 impact factor of 8.039, ranking it 1st out of 61 journals in the category "Chemistry, Medicinal".
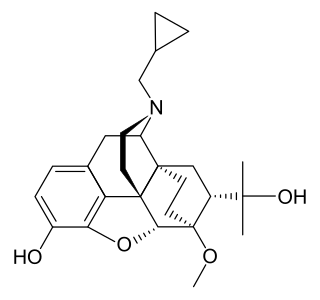
Diprenorphine, also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans.

7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as Kratom. It is often referred to as ‘7-OH’. It was first described in 1994 and is a natural product derived from the mitragynine present in the Kratom leaf. It is considered an oxidized derivative and active metabolite of mitragynine. 7-OH binds to opioid receptors like mitragynine, but research suggests that 7-OH binds with greater potency and contributes heavily to the analgesic activity of mitragynine as a metabolite.
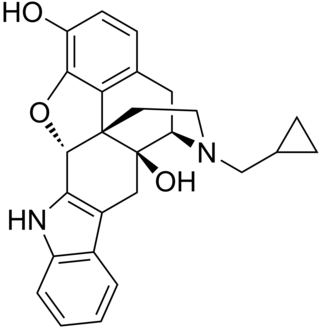
Naltrindole is a highly potent, highly selective delta opioid receptor antagonist used in biomedical research. In May 2012 a paper was published in Nature with the structure of naltrindole in complex with the mouse δ-opioid G-protein coupled receptor, solved by X-ray crystallography.

Allylprodine is an opioid analgesic that is an analog of prodine. It was discovered by Hoffman-La Roche in 1957 during research into the related drug pethidine. Derivatives were tested to prove the theory that phenolic & non-phenolic opioids bind at different sites of the opiate receptor.

SNC-80 is an opioid analgesic compound that selectively activates μ–δ opioid receptor heteromers and is used in scientific research. It was discovered in 1994.

(+)-BW373U86 is an opioid analgesic drug used in scientific research.

5'-Guanidinonaltrindole (5'-GNTI) is an opioid antagonist used in scientific research which is highly selective for the κ opioid receptor. It is 5x more potent and 500 times more selective than the commonly used κ-opioid antagonist norbinaltorphimine. It has a slow onset and long duration of action, and produces antidepressant effects in animal studies. It also increases allodynia by interfering with the action of the κ-opioid peptide dynorphin.
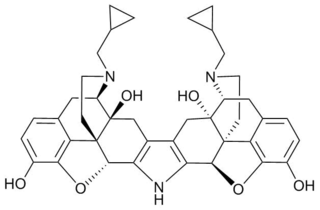
Norbinaltorphimine is an opioid antagonist used in scientific research. It is one of the few opioid antagonists available that is highly selective for the κ-opioid receptor, and blocks this receptor without affecting the μ- or δ-opioid receptors, although it has less selectivity in vivo than when used in isolated tissues. nor-BNI blocks the effects of κ-opioid agonists in animal models, and produces antidepressant and antipanic-like effects.

Xorphanol (INN), also known as xorphanol mesylate (USAN), is an opioid analgesic of the morphinan family that was never marketed.
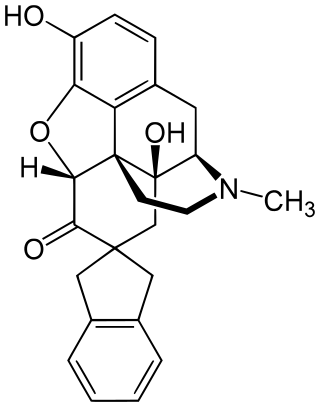
7-Spiroindanyloxymorphone (SIOM) is a drug that is used in scientific research. It is a selective δ-opioid agonist. It is a derivative of oxymorphone.
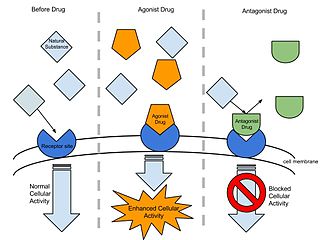
In pharmacology the term agonist-antagonist or mixed agonist/antagonist is used to refer to a drug which under some conditions behaves as an agonist while under other conditions, behaves as an antagonist.

Naltriben is a potent and selective antagonist for the delta opioid receptor, which is used in scientific research. It has similar effects to the more widely used δ antagonist naltrindole, but with different binding affinity for the δ1 and δ2 subtypes, which makes it useful for distinguishing the subtype selectivity of drugs acting at the δ receptors. It also acts as a κ-opioid agonist at high doses.

Chlornaltrexamine is an irreversible mixed agonist–antagonist for μ-opioid receptors, which forms a covalent bond to the binding site. It is 22 times more potent than morphine. Its alkylating group is a bis(chloroalkyl)amino-residue similar to that of the nitrogen mustards.

Dextrallorphan (DXA) is a chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.

N-Phenethylnormorphine is an opioid analgesic drug derived from morphine by replacing the N-methyl group with β-phenethyl. It is around eight to fourteen times more potent than morphine as a result of this modification, in contrast to most other N-substituted derivatives of morphine, which are substantially less active, or act as antagonists. Binding studies have helped to explain the increased potency of N-phenethylnormorphine, showing that the phenethyl group extends out to reach an additional binding point deeper inside the μ-opioid receptor cleft, analogous to the binding of the phenethyl group on fentanyl.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine. It is an analgesic being more potent than morphine.

beta-Fuoxymorphamine (β-fuoxymorphamine) is an opioid acting at μ-opioid receptors. It is used experimentally.
References
- ↑ "Philip Portoghese - COP - Medicinal Chemistry - University of Minnesota". Archived from the original on 2011-07-21. Retrieved 2011-06-07.
- ↑ "Editor". pubs.acs.org.
- ↑ Portoghese, Philip S.; Larson, Dennis L.; Sayre, Lawrence M.; Fries, David S.; Takemori, A. E. (1980). "A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities". Journal of Medicinal Chemistry. 23 (3): 233–234. doi:10.1021/jm00177a002. PMID 6245210.
- ↑ Portoghese, P.S.; Sultana, M.; Takemori, A.E. (1988). "Naltrindole, a highly selective and potent non-peptide δ opioid receptor antagonist". European Journal of Pharmacology. 146 (1): 185–186. doi:10.1016/0014-2999(88)90502-X. PMID 2832195.
- ↑ Portoghese, P. S.; Lipkowski, A. W.; Takemori, A. E. (1987). "Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists". Life Sciences. 40 (13): 1287–92. doi:10.1016/0024-3205(87)90585-6. PMID 2882399.
- ↑ Sofuoglu, M.; Portoghese, P. S.; Takemori, A. E. (1991). "Differential antagonism of delta opioid agonists by naltrindole and its benzofuran analog (NTB) in mice: Evidence for delta opioid receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics. 257 (2): 676–80. PMID 1851833.
- ↑ Erez, M.; Takemori, A. E.; Portoghese, P. S. (1982). "Narcotic antagonistic potency of bivalent ligands which contain .beta.-naltrexamine. Evidence for simultaneous occupation of proximal recognition sites". Journal of Medicinal Chemistry. 25 (7): 847–849. doi:10.1021/jm00349a016. PMID 7108900.
- ↑ Bhushan, Rashmi G.; Sharma, Shiv K.; Xie, Zhihua; Daniels, David J.; Portoghese, Philip S. (2004). "A Bivalent Ligand (KDN-21) Reveals Spinal δ and κ Opioid Receptors Are Organized as Heterodimers That Give Rise to δ1andκ2Phenotypes. Selective Targeting of δ−κ Heterodimers" (PDF). Journal of Medicinal Chemistry. 47 (12): 2969–2972. doi:10.1021/jm0342358. PMID 15163177.
- ↑ Daniels, D. J.; Lenard, N. R.; Etienne, C. L.; Law, P.-Y.; Roerig, S. C.; Portoghese, P. S. (2005). "Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series". Proceedings of the National Academy of Sciences. 102 (52): 19208–19213. Bibcode:2005PNAS..10219208D. doi: 10.1073/pnas.0506627102 . PMC 1323165 . PMID 16365317.
- ↑ "NIDA - Publications - NIDA Notes - Vol. 22, No. 1 - Innovations". Archived from the original on 2011-07-27. Retrieved 2011-06-07.
- ↑ "Philip S. Portoghese Symposium - College of Pharmacy - University of Minnesota". Archived from the original on 2011-07-21. Retrieved 2011-06-07.
- ↑ "ACS Division of Medicinal Chemistry".