Pyrimidine is an aromatic heterocyclic organic compound similar to pyridine. One of the three diazines, it has the nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine. In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U).
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).
Diphosphene is a type of organophosphorus compound that has a phosphorus–phosphorus double bond, denoted by R-P=P-R'. These compounds are not common but are of theoretical interest. Normally, compounds with the empirical formula RP exist as rings. However, like other multiple bonds between heavy main-group elements, P=P double bonds can be stabilized by a large steric hindrance from the substitutions. The first isolated diphosphene bis(2,4,6-tri-tert-butylphenyl)diphosphene was exemplified by Masaaki Yoshifuji and his coworkers in 1981, in which diphosphene is stabilized by two bulky phenyl group.

Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.
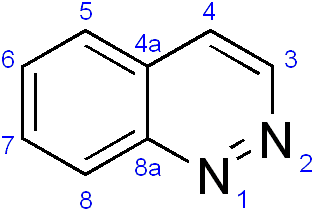
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline.
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.

Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula P4Sx with x ≤ 10. Two are of commercial significance, phosphorus pentasulfide (P4S10), which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide (P4S3), used in the production of "strike anywhere matches".
The Barton–Kellogg reaction is a coupling reaction between a diazo compound and a thioketone, giving an alkene by way of an episulfide intermediate. The Barton–Kellogg reaction is also known as Barton–Kellogg olefination and Barton olefin synthesis.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.
Phosphole is the organic compound with the chemical formula C
4H
4PH; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).

Tributylphosphine is the organophosphorus compound with the formula P(C
4H
9)
3. Abbreviated or PBu
3, it is a tertiary phosphine. It is an oily liquid at room temperature, with a nauseating odor. It reacts slowly with atmospheric oxygen, and rapidly with other oxidizing agents, to give the corresponding phosphine oxide. It is usually handled using air-free techniques.

Diphosphane, or diphosphine, is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air.

Woollins' reagent is an organic compound containing phosphorus and selenium. Analogous to Lawesson's reagent, it is used mainly as a selenation reagent. It is named after Professor John Derek Woollins.

Dichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of organophosphines.

Phospholane is the organophosphorus compound with the formula (CH2)4PH. This colorless liquid is the parent member of a family of five-membered, saturated rings containing phosphorus. Although phospholane itself is only of minor academic interest, the class of C- and P-substituted phospholanes are valued ligands in asymmetric hydrogenation and related areas of homogeneous catalysis. Phospholane is prepared by reduction of 1-chlorophospholane, which in turn is obtained by the reaction of 1-phenylphospholane and phosphorus trichloride.

Methyldichlorophosphine (alternatively known as dichloro(methyl)phosphane and methyl phosphonous dichloride) is an organophosphorus compound with the chemical formula CH3PCl2. It is a colorless, corrosive, flammable, and highly reactive liquid with a pungent odor.

Cyclopentaphosphine is the inorganic compound with the formula (PH)5. It is prepared by the hydrolysis of cyclo-[PSiMe3]4 (Me = methyl). Although only of theoretical interest, (PH)5 is parent of many related cyclic polyphosphines that are the subject of research.











