
Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible (400–750 nm), or infrared radiation (750–2500 nm).
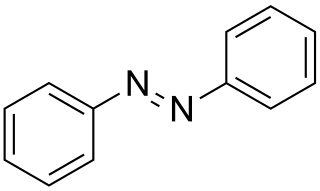
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings.

Resonance Raman spectroscopy is a variant of Raman spectroscopy in which the incident photon energy is close in energy to an electronic transition of a compound or material under examination. This similarity in energy (resonance) leads to greatly increased intensity of the Raman scattering of certain vibrational modes, compared to ordinary Raman spectroscopy.

An action spectrum is a graph of the rate of biological effectiveness plotted against wavelength of light. It is related to absorption spectrum in many systems. Mathematically, it describes the inverse quantity of light required to evoke a constant response. It is very rare for an action spectrum to describe the level of biological activity, since biological responses are often nonlinear with intensity.

A chromophore is a molecule which absorbs light at a particular wavelength and reflects color as a result. Chromophores are commonly referred to as colored molecules for this reason. The word is derived from Ancient Greek χρῶμᾰ (chroma) 'color' and -φόρος (phoros) 'carrier of'. Many molecules in nature are chromophores, including chlorophyll, the molecule responsible for the green colors of leaves. The color that is seen by our eyes is that of the light not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore indicates a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the moiety that causes a conformational change in the molecule when hit by light.
In particle physics, the quantum yield of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.

Photochromism is the reversible change of color upon exposure to light. It is a transformation of a chemical species (photoswitch) between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra.

Photosensitizers are light absorbers that alter the course of a photochemical reaction. They usually are catalysts. They can function by many mechanisms, sometimes they donate an electron to the substrate, sometimes they abstract a hydrogen atom from the substrate. At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons with one target molecule that dissociates into two fragments.
A light-harvesting complex consists of a number of chromophores which are complex subunit proteins that may be part of a larger super complex of a photosystem, the functional unit in photosynthesis. It is used by plants and photosynthetic bacteria to collect more of the incoming light than would be captured by the photosynthetic reaction center alone. The light which is captured by the chromophores is capable of exciting molecules from their ground state to a higher energy state, known as the excited state. This excited state does not last very long and is known to be short-lived.
The photosynthetic efficiency is the fraction of light energy converted into chemical energy during photosynthesis in green plants and algae. Photosynthesis can be described by the simplified chemical reaction

Photoinhibition is light-induced reduction in the photosynthetic capacity of a plant, alga, or cyanobacterium. Photosystem II (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the photosynthetic reaction center of PSII. Photoinhibition is also used in a wider sense, as dynamic photoinhibition, to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light.

A solar simulator is a device that provides illumination approximating natural sunlight. The purpose of the solar simulator is to provide a controllable indoor test facility under laboratory conditions. It can be used for the testing of any processes or materials that are photosensitive, including solar cells, sun screen, cosmetics, plastics, aerospace materials, skin cancer, bioluminescence, photosynthesis, water treatment, crude-oil degradation, and free radical formation. Solar simulators are used in a wide range of research areas including photobiology, photo-oxidation, photodegradation, photovoltaics, and photocatalysis.

Photon upconversion (UC) is a process in which the sequential absorption of two or more photons leads to the emission of light at shorter wavelength than the excitation wavelength. It is an anti-Stokes type emission. An example is the conversion of infrared light to visible light. Upconversion can take place in both organic and inorganic materials, through a number of different mechanisms. Organic molecules that can achieve photon upconversion through triplet-triplet annihilation are typically polycyclic aromatic hydrocarbons (PAHs). Inorganic materials capable of photon upconversion often contain ions of d-block or f-block elements. Examples of these ions are Ln3+, Ti2+, Ni2+, Mo3+, Re4+, Os4+, and so on.
The photoacoustic effect or optoacoustic effect is the formation of sound waves following light absorption in a material sample. In order to obtain this effect the light intensity must vary, either periodically or as a single flash. The photoacoustic effect is quantified by measuring the formed sound with appropriate detectors, such as microphones or piezoelectric sensors. The time variation of the electric output from these detectors is the photoacoustic signal. These measurements are useful to determine certain properties of the studied sample. For example, in photoacoustic spectroscopy, the photoacoustic signal is used to obtain the actual absorption of light in either opaque or transparent objects. It is useful for substances in extremely low concentrations, because very strong pulses of light from a laser can be used to increase sensitivity and very narrow wavelengths can be used for specificity. Furthermore, photoacoustic measurements serve as a valuable research tool in the study of the heat evolved in photochemical reactions, particularly in the study of photosynthesis.

Photogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition.
Christopher Barner-Kowollik FAA, FQA, FRSC, FRACI is an Australian Research Council (ARC) Laureate Fellow, the Senior Deputy Vice-Chancellor and Vice-President (Research) of the Queensland University of Technology (QUT) and Distinguished Professor within the School of Chemistry and Physics at the Queensland University of Technology (QUT) in Brisbane. From 2017 to 2024 he was Editor-in-Chief of the Royal Society of Chemistry (RSC) journal Polymer Chemistry, and is currently an editor for the RSC’s flagship journal Chemical Science. He is a principal investigator within the Soft Matter Materials Laboratory at QUT and associate research group leader at the Karlsruhe Institute of Technology (KIT).
Light harvesting materials harvest solar energy that can then be converted into chemical energy through photochemical processes. Synthetic light harvesting materials are inspired by photosynthetic biological systems such as light harvesting complexes and pigments that are present in plants and some photosynthetic bacteria. The dynamic and efficient antenna complexes that are present in photosynthetic organisms has inspired the design of synthetic light harvesting materials that mimic light harvesting machinery in biological systems. Examples of synthetic light harvesting materials are dendrimers, porphyrin arrays and assemblies, organic gels, biosynthetic and synthetic peptides, organic-inorganic hybrid materials, and semiconductor materials. Synthetic and biosynthetic light harvesting materials have applications in photovoltaics, photocatalysis, and photopolymerization.













