
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
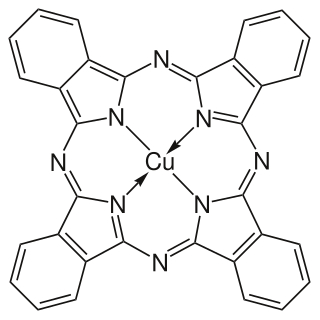
Copper phthalocyanine (CuPc), also called phthalocyanine blue, phthalo blue and many other names, is a bright, crystalline, synthetic blue pigment from the group of dyes based on phthalocyanines. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalis and acids. It has the appearance of a blue powder, insoluble in most solvents including water.

Azo compounds are organic compounds bearing the functional group diazenyl.

Quinacridone is an organic compound used as a pigment. Numerous derivatives constitute the quinacridone pigment family, which finds extensive use in industrial colorant applications such as robust outdoor paints, inkjet printer ink, tattoo inks, artists' watercolor paints, and color laser printer toner. As pigments, the quinacridones are insoluble. The development of this family of pigments supplanted the alizarin dyes.

Indian yellow is a complex pigment consisting primarily of euxanthic acid salts, euxanthone and sulphonated euxanthone. It is also known as purree, snowshoe yellow, gaugoli, gogili, Hardwari peori, Monghyr puri, peoli, peori, peri rung, pioury, piuri, purrea arabica, pwree, jaune indien, Indischgelb (German), yìndù huáng (Chinese), giallo indiano (Italian), amarillo indio (Spanish).

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.
In organic chemistry, an azo coupling is an reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon, serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.

Allura Red AC, also known as FD&C Red 40 or E129, is a red azo dye commonly used in food. It was developed in 1971 by the Allied Chemical Corporation, who gave the substance its name.

Lithol Rubine BK is a reddish synthetic azo dye. It has the appearance of a red powder and is magenta when printed. It is slightly soluble in hot water, insoluble in cold water, and insoluble in ethanol. When dissolved in dimethylformamide, its absorption maximum lies at about 442 nm. It is usually supplied as a calcium salt. It is prepared by azo coupling with 3-hydroxy-2-naphthoic acid. It is used to dye plastics, paints, printing inks, and for textile printing. It is normally used as a standard magenta in the three and four color printing processes.

Tattoo removal is the process of removing an unwanted tattoo. The process of tattooing generally creates permanent markings in the skin, but people have attempted many methods to try to hide or destroy tattoos.
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesics and dyes.
Arylide yellow, also known as Hansa yellow and monoazo yellow, is a family of organic compounds used as pigments. They are primarily used as industrial colorants including plastics, building paints and inks. They are also used in artistic oil paints, acrylics and watercolors. These pigments are usually semi-transparent and range from orange-yellow to yellow-greens. Related organic pigments are the diarylide pigments. Overall, these pigments have partially displaced the toxic cadmium yellow in the marketplace. Painters such as Alexander Calder and Jackson Pollock are known to have employed arylide yellow in their artworks.

Tattoo inks consist of pigments combined with a carrier, used in the process of tattooing to create a tattoo in the skin. These inks are also used for permanent makeup, a form of tattoo.
A variety of health effects can result from tattooing. Because it requires breaking the skin barrier, tattooing carries inherent health risks, including infection and allergic reactions. Modern tattooists reduce such risks by following universal precautions, working with single-use disposable needles, and sterilising equipment after each use. Many jurisdictions require tattooists to undergo periodic bloodborne pathogen training, such as is provided through the Red Cross and the U.S. Occupational Safety and Health Administration.

Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows, one example being Pigment Yellow 74.
Pigment Yellow 13 is an organic compound and an azo compound. It is a widely used yellow pigment. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Yellow 12, wherein the two xylyl groups are replaced by phenyl. It is often depicted as an azo (-N=N-) structure, but according to X-ray crystallography closely related compounds exist as the keto-hydrazide tautomers.
Pigment Orange 13 is an organic compound and an azo compound. It is a commercial orange pigment. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Orange 34, wherein the two phenyl groups are replaced by p-tolyl groups. Its structure has been confirmed by X-ray crystallography.

Toluidine red is an organic compound with the formula C10H6(OH)(N2C6H3 CH3). A dark red solid, the compound is classified as a azo dye consisting of a 2-naphthol group linked to a 2-nitro-4-methylphenyl substituent. Toluidine red is a traditional pigment, found in oil paints. Although once popular, it suffers as a pigment owing to "insufficient lightfastness and bleeding when incorporated into a paint system."

Pigment Orange 34 is an organic compound and an azo compound. It is a commercial orange pigment, i.e. an insoluble colorant. It is also classified as a diarylide pigment, being derived from 3,3'-dichlorobenzidine. It is closely related to Pigment Orange 13, wherein the two tolyl groups are replaced by phenyl groups.














