
Polyethylene or polythene is the most common plastic in use today. It is a polymer, primarily used for packaging. As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

A thermoplastic, or thermosoft plastic, is a plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.

Polyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one of the most studied conducting polymers.

In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained by copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively.

Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.
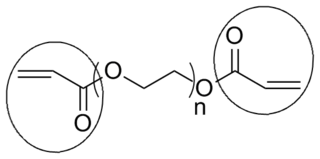
End groups are an important aspect of polymer synthesis and characterization. In polymer chemistry, end groups are functionalities or constitutional units that are at the extremity of a macromolecule or oligomer (IUPAC). In polymer synthesis, like condensation polymerization and free-radical types of polymerization, end-groups are commonly used and can be analyzed for example by nuclear magnetic resonance (NMR) to determine the average length of the polymer. Other methods for characterization of polymers where end-groups are used are mass spectrometry and vibrational spectrometry, like infrared and Raman spectrometry. Not only are these groups important for the analysis of the polymer, but they are also useful for grafting to and from a polymer chain to create a new copolymer. One example of an end group is in the polymer poly(ethylene glycol) diacrylate where the end-groups are circled.
Free-radical polymerization (FRP) is a method of polymerization, by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.
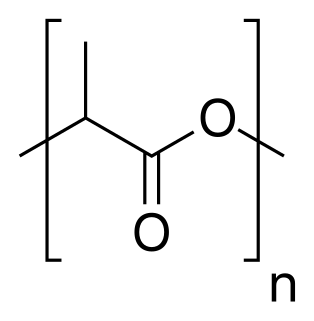
Polylactic acid, also known as poly(lactic acid) or polylactide is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent in the form of a thiocarbonylthio compound to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical polymerization techniques, others being atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthates, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed with conditions to favor low dispersity and a pre-chosen molecular weight. RAFT polymerization can be used to design polymers of complex architectures, such as linear block copolymers, comb-like, star, brush polymers, dendrimers and cross-linked networks.
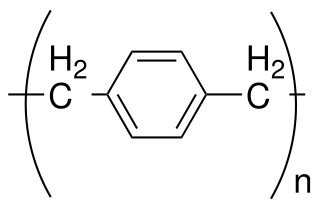
Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings –C
6H
4– connected by 1,2-ethanediyl bridges –CH
2–CH
2–. It can be obtained by polymerization of para-xylyleneH
2C=C
6H
4=CH
2.
In polymer chemistry, in situ polymerization is a preparation method that occurs "in the polymerization mixture" and is used to develop polymer nanocomposites from nanoparticles. There are numerous unstable oligomers (molecules) which must be synthesized in situ for use in various processes. The in situ polymerization process consists of an initiation step followed by a series of polymerization steps, which results in the formation of a hybrid between polymer molecules and nanoparticles. Nanoparticles are initially spread out in a liquid monomer or a precursor of relatively low molecular weight. Upon the formation of a homogenous mixture, initiation of the polymerization reaction is carried out by addition of an adequate initiator, which is exposed to a source of heat, radiation, etc. After the polymerization mechanism is completed, a nanocomposite is produced, which consists of polymer molecules bound to nanoparticles.
Plasma polymerization uses plasma sources to generate a gas discharge that provides energy to activate or fragment gaseous or liquid monomer, often containing a vinyl group, in order to initiate polymerization. Polymers formed from this technique are generally highly branched and highly cross-linked, and adhere to solid surfaces well. The biggest advantage to this process is that polymers can be directly attached to a desired surface while the chains are growing, which reduces steps necessary for other coating processes such as grafting. This is very useful for pinhole-free coatings of 100 picometers to 1 micrometre thickness with solvent insoluble polymers.
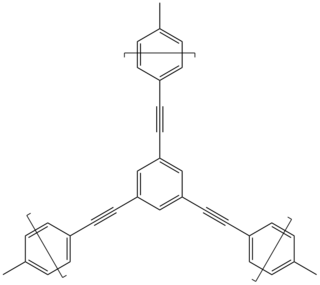
Conjugated microporous polymers (CMPs) are a sub-class of porous materials that are related to structures such as zeolites, metal-organic frameworks, and covalent organic frameworks, but are amorphous in nature, rather than crystalline. CMPs are also a sub-class of conjugated polymers and possess many of the same properties such as conductivity, mechanical rigidity, and insolubility. CMPs are created through the linking of building blocks in a π-conjugated fashion and possess 3-D networks. Conjugation extends through the system of CMPs and lends conductive properties to CMPs. Building blocks of CMPs are attractive in that the blocks possess broad diversity in the π units that can be used and allow for tuning and optimization of the skeleton and subsequently the properties of CMPs. Most building blocks have rigid components such as alkynes that cause the microporosity. CMPs have applications in gas storage, heterogeneous catalysis, light emitting, light harvesting, and electric energy storage.
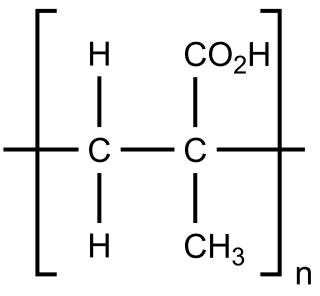
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid, which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a viscous liquid with a pungent odour. The first polymeric form of methacrylic acid was described in 1880 by Engelhorn and Fittig. The use of high purity monomers is required for proper polymerization conditions and therefore it is necessary to remove any inhibitors by extraction or via distillation. To prevent inhibition by dissolved oxygen, monomers should be carefully degassed prior to the start of the polymerization.

Star-shaped polymers are the simplest class of branched polymers with a general structure consisting of several linear chains connected to a central core. The core, or the center, of the polymer can be an atom, molecule, or macromolecule; the chains, or "arms", consist of variable-length organic chains. Star-shaped polymers in which the arms are all equivalent in length and structure are considered homogeneous, and ones with variable lengths and structures are considered heterogeneous.

In polymer chemistry, graft polymers are segmented copolymers with a linear backbone of one composite and randomly distributed branches of another composite. The picture labeled "graft polymer" shows how grafted chains of species B are covalently bonded to polymer species A. Although the side chains are structurally distinct from the main chain, the individual grafted chains may be homopolymers or copolymers. Graft polymers have been synthesized for many decades and are especially used as impact resistant materials, thermoplastic elastomers, compatibilizers, or emulsifiers for the preparation of stable blends or alloys. One of the better-known examples of a graft polymer is high impact polystyrene, which consists of a polystyrene backbone with polybutadiene grafted chains.
An organic radical battery (ORB) is a type of battery first developed in 2005. As of 2011, this type of battery was generally not available for the consumer, although their development at that time was considered to be approaching practical use. ORBs are potentially more environmentally friendly than conventional metal-based batteries, because they use organic radical polymers to provide electrical power instead of metals. ORBs are considered to be a high-power alternative to the Li-ion battery. Functional prototypes of the battery have been researched and developed by different research groups and corporations including the Japanese corporation NEC.

Allyl glycidyl ether is an organic compound used in adhesives and sealants and as a monomer for polymerization reactions. It is formally the condensation product of allyl alcohol and glycidol via an ether linkage. Because it contains both an alkene and an epoxide group, either group can be reacted selectively to yield a product where the other functional group remains intact for future reactions.















