
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol, which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary, secondary and tertiary alcohols.

Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitute 1% of all described fungal species.

Brettanomyces is a non-spore forming genus of yeast in the family Saccharomycetaceae, and is often colloquially referred to as "Brett". The genus name Dekkera is used interchangeably with Brettanomyces, as it describes the teleomorph or spore forming form of the yeast, but is considered deprecated under the one fungus, one name change. The cellular morphology of the yeast can vary from ovoid to long "sausage" shaped cells. The yeast is acidogenic, and when grown on glucose rich media under aerobic conditions, produces large amounts of acetic acid. Brettanomyces is important to both the brewing and wine industries due to the sensory compounds it produces.

Ferulic acid is a hydroxycinnamic acid, an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans.
A wine fault or defect is an unpleasant characteristic of a wine often resulting from poor winemaking practices or storage conditions, and leading to wine spoilage. Many of the compounds that cause wine faults are already naturally present in wine but at insufficient concentrations to be of issue. In fact, depending on perception, these concentrations may impart positive characters to the wine. However, when the concentration of these compounds greatly exceeds the sensory threshold, they replace or obscure the flavors and aromas that the wine should be expressing. Ultimately the quality of the wine is reduced, making it less appealing and sometimes undrinkable.
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups at various positions relative to the hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl groups in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.

3-Methylbutanoic acid, also known as β-methylbutyric acid or more commonly isovaleric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. The compound occurs naturally and can be found in many foods, such as cheese, soy milk, and apple juice.
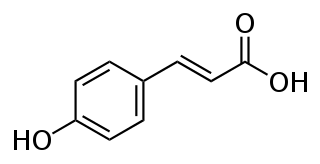
p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.
4-Ethylphenol (4-EP) is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A white solid, it occurs as an impurity in xylenols and as such is used in the production of some commercial phenolic resins. It is also a precursor to 4-vinylphenol.

4-Ethylguaiacol, often abbreviated to 4-EG, is a phenolic compound with the molecular formula C9H12O2. It can be produced in wine and beer by Brettanomyces. It is also frequently present in bio-oil produced by pyrolysis of lignocellulosic biomass.

Gentisic acid is a dihydroxybenzoic acid. It is a derivative of benzoic acid and a minor (1%) product of the metabolic break down of aspirin, excreted by the kidneys.

2-Methoxy-4-vinylphenol is an aromatic substance used as a flavoring agent. It is one of the compounds responsible for the natural aroma of buckwheat.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

In winemaking, clarification and stabilization are the processes by which insoluble matter suspended in the wine is removed before bottling. This matter may include dead yeast cells (lees), bacteria, tartrates, proteins, pectins, various tannins and other phenolic compounds, as well as pieces of grape skin, pulp, stems and gums. Clarification and stabilization may involve fining, filtration, centrifugation, flotation, refrigeration, pasteurization, and/or barrel maturation and racking.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

Wine is a complex mixture of chemical compounds in a hydro-alcoholic solution with a pH around 4. The chemistry of wine and its resultant quality depend on achieving a balance between three aspects of the berries used to make the wine: their sugar content, acidity and the presence of secondary compounds. Vines store sugar in grapes through photosynthesis, and acids break down as grapes ripen. Secondary compounds are also stored in the course of the season. Anthocyanins give grapes a red color and protection against ultraviolet light. Tannins add bitterness and astringency which acts to defend vines against pests and grazing animals.
Vinylphenol reductase is an enzyme that catalyses the reaction :

Phloretic acid is an organic compound with the formula HOC6H4(CH2)2CO2H. It is a white solid. The compound contains both phenol and carboxylic acid functional groups. It is sometimes called Desaminotyrosine (DAT) because it is identical to the common alpha amino acid tyrosine except for the absence of the amino functional group on the alpha carbon.
4-Hydroxycinnamate decarboxylase is an enzyme that uses p-coumaric acid to produce 4-ethylphenol.
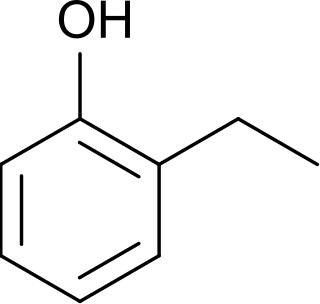
2-Ethylphenol is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A colorless liquid, it occurs as an impurity in xylenols and as such is used in the production of commercial phenolic resins. It is produced by ethylation of phenol using ethylene or ethanol in the presence of aluminium phenolate.















