Related Research Articles

The cell is the basic structural and functional unit of life forms. Every cell consists of a cytoplasm enclosed within a membrane, which contains many biomolecules such as proteins and nucleic acids.

A flagellum is a hairlike appendage that protrudes from certain plant and animal sperm cells, and from a wide range of microorganisms to provide motility. Many protists with flagella are termed as flagellates.

ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). It is classified under ligases as it changes ADP by the formation of P-O bond (phosphodiester bond). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is:

Vibrio is a genus of Gram-negative bacteria, possessing a curved-rod (comma) shape, several species of which can cause foodborne infection, usually associated with eating undercooked seafood. Being highly salt tolerant and unable to survive in fresh water, Vibrio spp. are commonly found in various salt water environments. Vibrio spp. are facultative anaerobes that test positive for oxidase and do not form spores. All members of the genus are motile. They are able to have polar or lateral flagellum with or without sheaths. Vibrio species typically possess two chromosomes, which is unusual for bacteria. Each chromosome has a distinct and independent origin of replication, and are conserved together over time in the genus. Recent phylogenies have been constructed based on a suite of genes.

Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels and can be classified according to the trigger that opens the channel for such ions, i.e. either a voltage-change ("voltage-gated", "voltage-sensitive", or "voltage-dependent" sodium channel; also called "VGSCs" or "Nav channel") or a binding of a substance (a ligand) to the channel (ligand-gated sodium channels).
Bacterial display is a protein engineering technique used for in vitro protein evolution. Libraries of polypeptides displayed on the surface of bacteria can be screened using flow cytometry or iterative selection procedures (biopanning). This protein engineering technique allows us to link the function of a protein with the gene that encodes it. Bacterial display can be used to find target proteins with desired properties and can be used to make affinity ligands which are cell-specific. This system can be used in many applications including the creation of novel vaccines, the identification of enzyme substrates and finding the affinity of a ligand for its target protein.
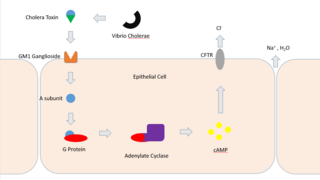
Cholera toxin is AB5 multimeric protein complex secreted by the bacterium Vibrio cholerae. CTX is responsible for the massive, watery diarrhea characteristic of cholera infection. It is a member of the Heat-labile enterotoxin family.
The sodium/phosphate cotransporter is a member of the phosphate:Na+ symporter (PNaS) family within the TOG Superfamily of transport proteins as specified in the Transporter Classification Database (TCDB).

Multidrug and toxin extrusion protein 2 is a protein which in humans is encoded by the SLC47A2 gene.

Bacterial motility is the ability of bacteria to move independently using metabolic energy. Most motility mechanisms which evolved among bacteria also evolved in parallel among the archaea. Most rod-shaped bacteria can move using their own power, which allows colonization of new environments and discovery of new resources for survival. Bacterial movement depends not only on the characteristics of the medium, but also on the use of different appendages to propel. Swarming and swimming movements are both powered by rotating flagella. Whereas swarming is a multicellular 2D movement over a surface and requires the presence of surfactants, swimming is movement of individual cells in liquid environments.

Several organisms are capable of rolling locomotion. However, true wheels and propellers—despite their utility in human vehicles—do not seem to play a significant role in the movement of living things. Biologists have offered several explanations for the apparent absence of biological wheels, and wheeled creatures have appeared often in speculative fiction.
Multi-antimicrobial extrusion protein (MATE) also known as multidrug and toxin extrusion or multidrug and toxic compound extrusion is a family of proteins which function as drug/sodium or proton antiporters.
Motility protein A, MotA, is a bacterial protein that is encoded by the motA gene. It is a component of the flagellar motor. More specifically, MotA and MotB make the stator of a H+ driven bacterial flagella and surround the rotor as a ring of about 8–10 particles. MotA and MotB are integral membrane proteins. MotA has four transmembrane domains.
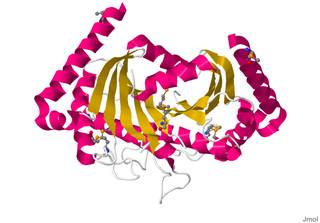
Motility protein B also known as MotB is a bacterial protein that is encoded by the motB gene. It's a component of the flagellar motor. More specifically, MotA and MotB makes the stator of a flagellum and surround the rotor as a ring of about 8-10 particles. MotA and MotB are integral membrane proteins. While both MotA and MotB surround the MS ring, MotB also anchors MotA to cell wall peptidoglycan. These two proteins form pores that harvest energy for flagellar mechanical movement by proton motive force (PMF) across the membrane. Cellular metabolic processes such as the electron transport chain move protons outside the cell, creating more protons and more positive charge in the extracellular space. When the protons flow back into the cell through MotA and MotB along concentration and charge gradients, they release energy that is used for flagellar rotation. The speed of the flagellar motor is dependent on the magnitude of the PMF acting on MotA and MotB.
The CTXφ bacteriophage is a filamentous bacteriophage. It is a positive-strand DNA virus with single-stranded DNA (ssDNA).

In molecular biology, the OmpA domain is a conserved protein domain with a beta/alpha/beta/alpha-beta(2) structure found in the C-terminal region of many Gram-negative bacterial outer membrane proteins, such as porin-like integral membrane proteins, small lipid-anchored proteins, and MotB proton channels. The N-terminal half of these proteins is variable although some of the proteins in this group have the OmpA-like transmembrane domain at the N terminus. OmpA from Escherichia coli is required for pathogenesis, and can interact with host receptor molecules. MotB serve two functions in E. coli, the MotA(4)-MotB(2) complex attaches to the cell wall via MotB to form the stator of the flagellar motor, and the MotA-MotB complex couples the flow of ions across the cell membrane to movement of the rotor.
The NhaB family belongs to the ion transporter (IT) superfamily. A representative list of proteins belonging to the NhaB family can be found in the Transporter Classification Database.
PomB is a protein that is part of the stator in Na+ driven bacterial flagella. Na influx to torque generation in the polar flagellar motor of Vibrio alginolyticus. The stator complex is fixed-anchored around the rotor through a putative peptidoglycan-binding (PGB) domain in the periplasmic region of PomB.

Bacterial secretion systems are protein complexes present on the cell membranes of bacteria for secretion of substances. Specifically, they are the cellular devices used by pathogenic bacteria to secrete their virulence factors to invade the host cells. They can be classified into different types based on their specific structure, composition and activity. Generally, proteins can be secreted through two different processes. One process is a one-step mechanism in which proteins from the cytoplasm of bacteria are transported and delivered directly through the cell membrane into the host cell. Another involves a two-step activity in which the proteins are first transported out of the inner cell membrane, then deposited in the periplasm, and finally through the outer cell membrane into the host cell.
References
- ↑ Asai, Yukako; Kawagishi, Ikuro; Sockett, R. Elizabeth; Homma, Michio (October 1999). "Hybrid motor with H+- and Na+-driven components can rotate Vibrio polar flagella by using sodium ions". Journal of Bacteriology. 181 (20): 6332–38. doi:10.1128/JB.181.20.6332-6338.1999. PMC 103767 . PMID 10515922.