
Potassium is a chemical element; it has symbol K and atomic number 19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge. In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-colored flame. It is found dissolved in seawater, and occurs in many minerals such as orthoclase, a common constituent of granites and other igneous rocks.

Potassium chloride is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners, and in food processing, where it may be known as E number additive E508.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Potassium bicarbonate (IUPAC name: potassium hydrogencarbonate, also known as potassium acid carbonate) is the inorganic compound with the chemical formula KHCO3. It is a white solid.
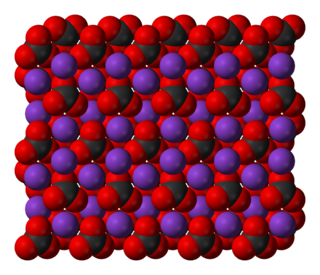
Potassium carbonate is the inorganic compound with the formula K2CO3. It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass. Commonly, it can be found as the result of leakage of alkaline batteries.

Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and MnO−
4, an intensely pink to purple solution.

Potassium cyanide is a compound with the formula KCN. It is a colorless salt, similar in appearance to sugar, that is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewellery for chemical gilding and buffing. Potassium cyanide is highly toxic, and a dose of 200 to 300 milligrams will kill nearly any human.
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. The reaction was reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912.
In organic chemistry, a group transfer reaction is a class of the pericyclic reaction where one or more groups of atoms is transferred from one molecule to another. Group transfer reactions can sometimes be difficult to identify when separate reactant molecules combine into a single product molecule. Unlike other pericyclic reaction classes, group transfer reactions do not have a specific conversion of pi bonds into sigma bonds or vice versa, and tend to be less frequently encountered. Like all pericyclic reactions, group transfer reactions must obey the Woodward–Hoffmann rules. Group transfer reactions can be divided into two distinct subcategories: the ene reaction and the diimide reduction. Group transfer reactions have diverse applications in various fields, including protein adenylation, biocatalytic and chemoenzymatic approaches for chemical synthesis, and strengthening skim natural rubber latex.
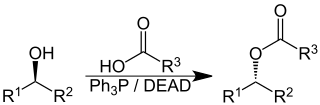
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). In a typical protocol, one dissolves the alcohol, the carboxylic acid, and triphenylphosphine in tetrahydrofuran or other suitable solvent, cool to 0 °C using an ice-bath, slowly add the DEAD dissolved in THF, then stir at room temperature for several hours. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion as the nucleophile displaces it. A common side-product is produced when the azodicarboxylate displaces the leaving group instead of the desired nucleophile. This happens if the nucleophile is not acidic enough or is not nucleophilic enough due to steric or electronic constraints. A variation of this reaction utilizing a nitrogen nucleophile is known as a Fukuyama–Mitsunobu.

Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2−O−C(=O)−N=N−C(=O)−O−CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles.
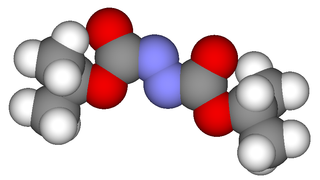
Diisopropyl azodicarboxylate (DIAD) is the diisopropyl ester of azodicarboxylic acid. It is used as a reagent in the production of many organic compounds. It is often used in the Mitsunobu reaction, where it serves as an oxidizer of triphenylphosphine to triphenylphosphine oxide. It has also been used to generate aza-Baylis-Hillman adducts with acrylates. It can also serve as a selective deprotectant of N-benzyl groups in the presence of other protecting groups.
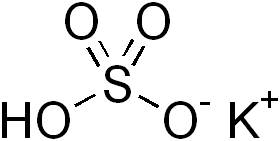
Potassium bisulfate (potassium bisulphate) is an inorganic compound with the chemical formula KHSO4 and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid.

Triazene is an unsaturated inorganic compound having the chemical formula N3H3. It has one double bond and is the second-simplest member of the azene class of hydronitrogen compounds, after diimide. Triazenes are a class of organic compounds containing the functional group -N(H)-N=N-. Triazene, possibly along with its isomer triimide (HNNHNH), has been synthesized in electron-irradiated ices of ammonia and ammonia/dinitrogen and detected in the gas phase after sublimation.
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene.
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the fluorine azide. It has the structure F−N=N−F and exists in both cis and trans isomers, as typical for diimides.
Reductions with diimide are a chemical reactions that convert unsaturated organic compounds to reduced alkane products. In the process, diimide is oxidized to dinitrogen.

A rylene dye is a dye based on the rylene framework of naphthalene units linked in peri-positions. In homologues additional naphthalene units are added, forming compounds — or poly(peri-naphthalene)s — such as perylene, terrylene and quarterrylene.

Sulfur diimides are chemical compounds of the formula S(NR)2. Structurally, they are the diimine of sulfur dioxide. The parent member, S(NH)2, is of only theoretical interest. Other derivatives where R is an organic group are stable and useful reagents.















