Related Research Articles

The hertz is the unit of frequency in the International System of Units (SI), often described as being equivalent to one event per second. The hertz is an SI derived unit whose formal expression in terms of SI base units is s−1, meaning that one hertz is one per second or the reciprocal of one second. It is used only in the case of periodic events. It is named after Heinrich Rudolf Hertz (1857–1894), the first person to provide conclusive proof of the existence of electromagnetic waves. For high frequencies, the unit is commonly expressed in multiples: kilohertz (kHz), megahertz (MHz), gigahertz (GHz), terahertz (THz).

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

The caesium standard is a primary frequency standard in which the photon absorption by transitions between the two hyperfine ground states of caesium-133 atoms is used to control the output frequency. The first caesium clock was built by Louis Essen in 1955 at the National Physical Laboratory in the UK and promoted worldwide by Gernot M. R. Winkler of the United States Naval Observatory.

A frequency standard is a stable oscillator used for frequency calibration or reference. A frequency standard generates a fundamental frequency with a high degree of accuracy and precision. Harmonics of this fundamental frequency are used to provide reference points.

A rubidium standard or rubidium atomic clock is a frequency standard in which a specified hyperfine transition of electrons in rubidium-87 atoms is used to control the output frequency.

A magnetometer is a device that measures magnetic field or magnetic dipole moment. Different types of magnetometers measure the direction, strength, or relative change of a magnetic field at a particular location. A compass is one such device, one that measures the direction of an ambient magnetic field, in this case, the Earth's magnetic field. Other magnetometers measure the magnetic dipole moment of a magnetic material such as a ferromagnet, for example by recording the effect of this magnetic dipole on the induced current in a coil.
In electromagnetism, the magnetic susceptibility is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization M to the applied magnetic field intensity H. This allows a simple classification, into two categories, of most materials' responses to an applied magnetic field: an alignment with the magnetic field, χ > 0, called paramagnetism, or an alignment against the field, χ < 0, called diamagnetism.
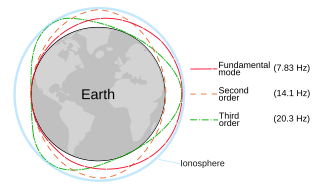
The Schumann resonances (SR) are a set of spectrum peaks in the extremely low frequency portion of the Earth's electromagnetic field spectrum. Schumann resonances are global electromagnetic resonances, generated and excited by lightning discharges in the cavity formed by the Earth's surface and the ionosphere.
Louis Essen OBE FRS(6 September 1908 – 24 August 1997) was an English physicist whose most notable achievements were in the precise measurement of time and the determination of the speed of light. He was a critic of Albert Einstein's theory of relativity, particularly as it related to time dilation.

A proton magnetometer, also known as a proton precession magnetometer (PPM), uses the principle of Earth's field nuclear magnetic resonance (EFNMR) to measure very small variations in the Earth's magnetic field, allowing ferrous objects on land and at sea to be detected.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field. This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increased proportionally to the strength of the external magnetic field. Notably, the resonance frequency of each NMR-active nucleus depends on its chemical environment. As a result, NMR spectra provide information about individual functional groups present in the sample, as well as about connections between nearby nuclei in the same molecule. As the NMR spectra are unique or highly characteristic to individual compounds and functional groups, NMR spectroscopy is one of the most important methods to identify molecular structures, particularly of organic compounds.

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.
Ferromagnetic resonance, or FMR, is coupling between an electromagnetic wave and the magnetization of a medium through which it passes. This coupling induces a significant loss of power of the wave. The power is absorbed by the precessing magnetization of the material and lost as heat. For this coupling to occur, the frequency of the incident wave must be equal to the precession frequency of the magnetization and the polarization of the wave must match the orientation of the magnetization.

An atomic line filter (ALF) is a more effective optical band-pass filter used in the physical sciences for filtering electromagnetic radiation with precision, accuracy, and minimal signal strength loss. Atomic line filters work via the absorption or resonance lines of atomic vapors and so may also be designated an atomic resonance filter (ARF).
In a chemical analysis, the internal standard method involves adding the same amount of a chemical substance to each sample and calibration solution. The internal standard responds proportionally to changes in the analyte and provides a similar, but not identical, measurement signal. It must also be absent from the sample matrix to ensure there is no other source of the internal standard present. Taking the ratio of analyte signal to internal standard signal and plotting it against the analyte concentrations in the calibration solutions will result in a calibration curve. The calibration curve can then be used to calculate the analyte concentration in an unknown sample.
Ion cyclotron resonance is a phenomenon related to the movement of ions in a magnetic field. It is used for accelerating ions in a cyclotron, and for measuring the masses of an ionized analyte in mass spectrometry, particularly with Fourier transform ion cyclotron resonance mass spectrometers. It can also be used to follow the kinetics of chemical reactions in a dilute gas mixture, provided these involve charged species.
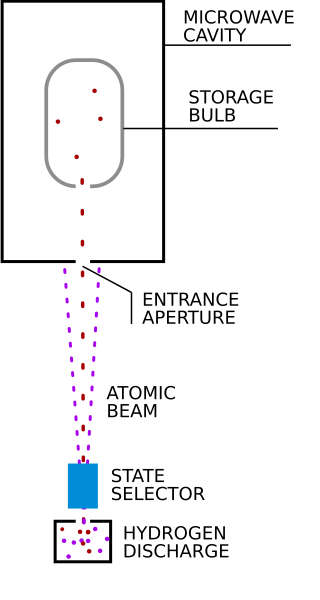
A hydrogen maser, also known as hydrogen frequency standard, is a specific type of maser that uses the intrinsic properties of the hydrogen atom to serve as a precision frequency reference.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. High-resolution nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). The original application of NMR to condensed matter physics is nowadays mostly devoted to strongly correlated electron systems. It reveals large many-body couplings by fast broadband detection and should not be confused with solid state NMR, which aims at removing the effect of the same couplings by Magic Angle Spinning techniques.

An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions between such states they interact with a very specific frequency of electromagnetic radiation. This phenomenon serves as the basis for the International System of Units' (SI) definition of a second:
The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency, , the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be 9192631770 when expressed in the unit Hz, which is equal to s−1.
Electromagnetically induced acoustic noise, electromagnetically excited acoustic noise, or more commonly known as coil whine, is audible sound directly produced by materials vibrating under the excitation of electromagnetic forces. Some examples of this noise include the mains hum, hum of transformers, the whine of some rotating electric machines, or the buzz of fluorescent lamps. The hissing of high voltage transmission lines is due to corona discharge, not magnetism.
References
 This article incorporates public domain material from Federal Standard 1037C. General Services Administration. Archived from the original on 2022-01-22. (in support of MIL-STD-188).
This article incorporates public domain material from Federal Standard 1037C. General Services Administration. Archived from the original on 2022-01-22. (in support of MIL-STD-188).