
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science.

Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Propane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is often a constituent of liquefied petroleum gas (LPG), which is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation; other constituents of LPG may include propylene, butane, butylene, butadiene, and isobutylene. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane has lower volumetric energy density than gasoline or coal, but has higher gravimetric energy density than them and burns more cleanly.

Stoichiometry is the relationship between the weights of reactants and products before, during, and following chemical reactions.

Producer gas is fuel gas that is manufactured by blowing through a coke or coal fire with air and steam simultaneously. It mainly consists of carbon monoxide (CO), hydrogen (H2), as well as substantial amounts of nitrogen (N2). The caloric value of the producer gas is low (mainly because of its high nitrogen content), and the technology is obsolete. Improvements over producer gas, also obsolete, include water gas where the solid fuel is treated intermittently with air and steam and, far more efficiently synthesis gas where the solid fuel is replaced with methane.
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.
The heating value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specified amount of it.

Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.

MAPP gas was a trademarked name, belonging to The Linde Group, a division of the former global chemical giant Union Carbide, for a fuel gas based on a stabilized mixture of methylacetylene (propyne), propadiene and propane. The name comes from the original chemical composition, methylacetylene-propadiene propane. "MAPP gas" is also widely used as a generic name for UN 1060 stabilised methylacetylene-propadiene.
Air–fuel ratio (AFR) is the mass ratio of air to a solid, liquid, or gaseous fuel present in a combustion process. The combustion may take place in a controlled manner such as in an internal combustion engine or industrial furnace, or may result in an explosion ,The air–fuel ratio determines whether a mixture is combustible at all, how much energy is being released, and how much unwanted pollutants are produced in the reaction. Typically a range of fuel to air ratios exists, outside of which ignition will not occur. These are known as the lower and upper explosive limits.

Oxyhydrogen is a mixture of hydrogen (H2) and oxygen (O2) gases. This gaseous mixture is used for torches to process refractory materials and was the first gaseous mixture used for welding. Theoretically, a ratio of 2:1 hydrogen:oxygen is enough to achieve maximum efficiency; in practice a ratio 4:1 or 5:1 is needed to avoid an oxidizing flame.
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer [coke] with air and gasifying it with steam". The caloric yield of the fuel produced by this method is about 10% of the yield from a modern syngas plant. The coke needed to produce water gas also costs significantly more than the precursors for syngas, making water gas technology an even less attractive business proposition.

A gas burner is a device that produces a non-controlled flame by mixing a fuel gas such as acetylene, natural gas, or propane with an oxidizer such as the ambient air or supplied oxygen, and allowing for ignition and combustion.
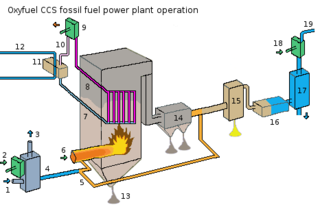
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.

Oxy-fuel welding and oxy-fuel cutting are processes that use fuel gases and oxygen to weld or cut metals. French engineers Edmond Fouché and Charles Picard became the first to develop oxygen-acetylene welding in 1903. Pure oxygen, instead of air, is used to increase the flame temperature to allow localized melting of the workpiece material in a room environment.

Butane or n-butane is an alkane with the formula C4H10. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature and pressure. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties, and commercialized by Walter O. Snelling in the early 1910s.

A blowtorch, also referred to as a blowlamp, is an ambient air fuel-burning tool used for applying flame and heat to various applications, usually in metalworking.
Flame treatment is the application of a gas flame to the surface of a material to improve adhesion.

















