Pseudomonas citronellolis is a Gram-negative, bacillus bacterium that is used to study the mechanisms of pyruvate carboxylase. It was first isolated from forest soil, under pine trees, in northern Virginia, United States.
Pseudomonas mendocina is a Gram-negative environmental bacterium that can cause opportunistic infections, such as infective endocarditis and spondylodiscitis, although cases are very rare. It has potential use in bioremediation as it is able to degrade toluene. Based on 16S rRNA analysis, P. mendocina has been placed in the P. aeruginosa group.
Pseudomonas taetrolens is a Gram-negative, nonsporulating, motile, rod-shaped bacterium that causes mustiness in eggs. Based on 16S rRNA analysis, P. taetrolens has been placed in the P. chlororaphis group.
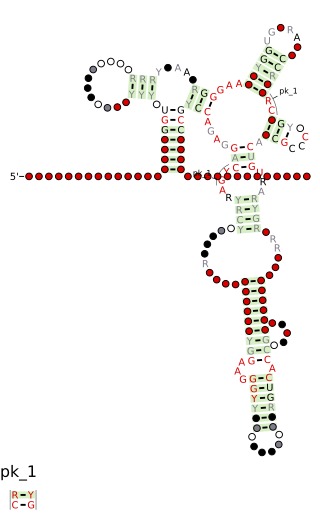
Cobalamin riboswitch is a cis-regulatory element which is widely distributed in 5' untranslated regions of vitamin B12 (Cobalamin) related genes in bacteria.

In enzymology, a precorrin-2 C20-methyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, precorrin-3B C17-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-4 C11-methyltransferase is an enzyme that catalyzes the chemical reaction
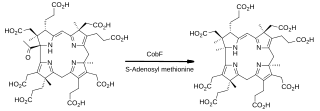
In enzymology, precorrin-6A synthase (deacetylating) (EC 2.1.1.152) is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-6Y C5,15-methyltransferase (decarboxylating) (EC 2.1.1.132) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-6A reductase (EC 1.3.1.54) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-3B synthase (EC 1.14.13.83) is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-8X methylmutase is an enzyme that catalyzes the chemical reaction

Cobalt chelatase (EC 6.6.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a hydrogenobyrinic acid a,c-diamide synthase (glutamine-hydrolysing) (EC 6.3.5.9) is an enzyme that catalyzes the chemical reaction

Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. It is one of eight B vitamins. It is required by animals, which use it as a cofactor in DNA synthesis, and in both fatty acid and amino acid metabolism. It is important in the normal functioning of the nervous system via its role in the synthesis of myelin, and in the circulatory system in the maturation of red blood cells in the bone marrow. Plants do not need cobalamin and carry out the reactions with enzymes that are not dependent on it.

In enzymology, a nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

In molecular biology, cob(I)yrinic acid a,c-diamide adenosyltransferase EC 2.5.1.17 is an enzyme which catalyses the conversion of cobalamin into one of its coenzyme forms, adenosylcobalamin. Adenosylcobalamin is required as a cofactor for the activity of certain enzymes. AdoCbl contains an adenosyl moiety liganded to the cobalt ion of cobalamin via a covalent Co-C bond.

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.
Adenosylcobinamide-GDP ribazoletransferase is an enzyme with systematic name adenosylcobinamide-GDP:alpha-ribazole ribazoletransferase. This enzyme catalyses the following chemical reaction
Shimwellia blattae is a species of bacterium, one of two in the genus Shimwellia. It is an aerobic enteric bacterium first isolated from the hindgut of cockroaches. Although it is related to human pathogens, including Escherichia coli, S. blattae is not pathogenic to humans. It is notable for its ability to synthesize vitamin B12 de novo.










