
A blood cell is a cell produced through hematopoiesis and found mainly in the blood. Major types of blood cells include red blood cells (erythrocytes), white blood cells (leukocytes), and platelets (thrombocytes). Together, these three kinds of blood cells add up to a total 45% of the blood tissue by volume, with the remaining 55% of the volume composed of plasma, the liquid component of blood.

An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.

Platelets or thrombocytes are a blood component whose function is to react to bleeding from blood vessel injury by clumping, thereby initiating a blood clot. Platelets have no cell nucleus; they are fragments of cytoplasm derived from the megakaryocytes of the bone marrow or lung, which then enter the circulation. Platelets are found only in mammals, whereas in other vertebrates, thrombocytes circulate as intact mononuclear cells.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.
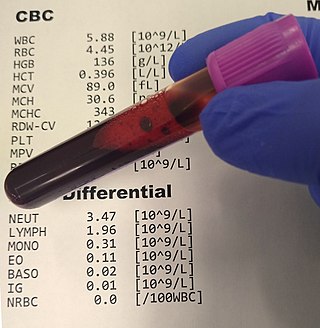
A complete blood count (CBC), also known as a full blood count (FBC), is a set of medical laboratory tests that provide information about the cells in a person's blood. The CBC indicates the counts of white blood cells, red blood cells and platelets, the concentration of hemoglobin, and the hematocrit. The red blood cell indices, which indicate the average size and hemoglobin content of red blood cells, are also reported, and a white blood cell differential, which counts the different types of white blood cells, may be included.

An automated analyser is a medical laboratory instrument designed to measure various substances and other characteristics in a number of biological samples quickly, with minimal human assistance. These measured properties of blood and other fluids may be useful in the diagnosis of disease.

A blood bank is a center where blood gathered as a result of blood donation is stored and preserved for later use in blood transfusion. The term "blood bank" typically refers to a department of a hospital usually within a clinical pathology laboratory where the storage of blood product occurs and where pre-transfusion and blood compatibility testing is performed. However, it sometimes refers to a collection center, and some hospitals also perform collection. Blood banking includes tasks related to blood collection, processing, testing, separation, and storage.

A blood smear, peripheral blood smear or blood film is a thin layer of blood smeared on a glass microscope slide and then stained in such a way as to allow the various blood cells to be examined microscopically. Blood smears are examined in the investigation of hematological (blood) disorders and are routinely employed to look for blood parasites, such as those of malaria and filariasis.
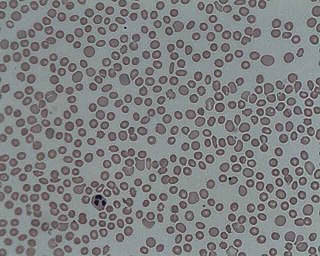
In hematology, thrombocytopenia is a condition characterized by abnormally low levels of platelets in the blood. Low levels of platelets in turn may lead to prolonged or excessive bleeding. It is the most common coagulation disorder among intensive care patients and is seen in a fifth of medical patients and a third of surgical patients.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.

Heparin-induced thrombocytopenia (HIT) is the development of thrombocytopenia, due to the administration of various forms of heparin, an anticoagulant. HIT predisposes to thrombosis. When thrombosis is identified the condition is called heparin-induced thrombocytopenia and thrombosis (HITT). HIT is caused by the formation of abnormal antibodies that activate platelets, which release microparticles that activate thrombin, leading to thrombosis. If someone receiving heparin develops new or worsening thrombosis, or if the platelet count falls, HIT can be confirmed with specific blood tests.

The partial thromboplastin time (PTT), also known as the activated partial thromboplastin time, is a blood test that characterizes coagulation of the blood. A historical name for this measure is the kaolin-cephalin clotting time (KCCT), reflecting kaolin and cephalin as materials historically used in the test. Apart from detecting abnormalities in blood clotting, partial thromboplastin time is also used to monitor the treatment effect of heparin, a widely prescribed drug that reduces blood's tendency to clot.

Plateletpheresis is the process of collecting thrombocytes, more commonly called platelets, a component of blood involved in blood clotting. The term specifically refers to the method of collecting the platelets, which is performed by a device used in blood donation that separates the platelets and returns other portions of the blood to the donor. Platelet transfusion can be a life-saving procedure in preventing or treating serious complications from bleeding and hemorrhage in patients who have disorders manifesting as thrombocytopenia or platelet dysfunction. This process may also be used therapeutically to treat disorders resulting in extraordinarily high platelet counts such as essential thrombocytosis.
Evans syndrome is an autoimmune disease in which an individual's immune system attacks their own red blood cells and platelets, the syndrome can include immune neutropenia. These immune cytopenias may occur simultaneously or sequentially.
Mean platelet volume (MPV) is a machine-calculated measurement of the average size of platelets found in blood and is typically included in blood tests as part of the CBC. Since the average platelet size is larger when the body is producing increased numbers of platelets, the MPV test results can be used to make inferences about platelet production in bone marrow or platelet destruction problems.

Ristocetin is a glycopeptide antibiotic, obtained from Amycolatopsis lurida, previously used to treat staphylococcal infections. It is no longer used clinically because it caused thrombocytopenia and platelet agglutination. It is now used solely to assay those functions in vitro in the diagnosis of conditions such as von Willebrand disease (vWD) and Bernard–Soulier syndrome. Platelet agglutination caused by ristocetin can occur only in the presence of von Willebrand factor multimers, so if ristocetin is added to blood lacking the factor, the platelets will not clump.
Pseudo gray platelet syndrome was described by Cockbill, Burmester, and Heptinstall (1988) who reported a 25-year-old woman with a history of mild bruising and bleeding. Another case was described in Japan in 2002.

A white blood cell differential is a medical laboratory test that provides information about the types and amounts of white blood cells in a person's blood. The test, which is usually ordered as part of a complete blood count (CBC), measures the amounts of the five normal white blood cell types – neutrophils, lymphocytes, monocytes, eosinophils and basophils – as well as abnormal cell types if they are present. These results are reported as percentages and absolute values, and compared against reference ranges to determine whether the values are normal, low, or high. Changes in the amounts of white blood cells can aid in the diagnosis of many health conditions, including viral, bacterial, and parasitic infections and blood disorders such as leukemia.

Post-vaccination embolic and thrombotic events, termed vaccine-induced immune thrombotic thrombocytopenia (VITT), vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), thrombosis with thrombocytopenia syndrome (TTS), vaccine-induced immune thrombocytopenia and thrombosis (VITT), or vaccine-associated thrombotic thrombocytopenia (VATT), are rare types of blood clotting syndromes that were initially observed in a number of people who had previously received the Oxford–AstraZeneca COVID‑19 vaccine (AZD1222) during the COVID‑19 pandemic. It was subsequently also described in the Janssen COVID‑19 vaccine, leading to the suspension of its use until its safety had been reassessed. On 5 May 2022 the FDA posted a bulletin limiting the use of the Janssen Vaccine to very specific cases due to further reassessment of the risks of TTS, although the FDA also stated in the same bulletin that the benefits of the vaccine outweigh the risks.

















