
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved G domain common to many GTPases.

Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are part of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the plasma membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus.
The Rab family of proteins is a member of the Ras superfamily of small G proteins. Approximately 70 types of Rabs have now been identified in humans. Rab proteins generally possess a GTPase fold, which consists of a six-stranded beta sheet which is flanked by five alpha helixes. Rab GTPases regulate many steps of membrane trafficking, including vesicle formation, vesicle movement along actin and tubulin networks, and membrane fusion. These processes make up the route through which cell surface proteins are trafficked from the Golgi to the plasma membrane and are recycled. Surface protein recycling returns proteins to the surface whose function involves carrying another protein or substance inside the cell, such as the transferrin receptor, or serves as a means of regulating the number of a certain type of protein molecules on the surface.

The gene EEA1 encodes for the 1400 amino acid protein, Early Endosome Antigen 1.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.
Ras-related protein Rab-5A is a protein that in humans is encoded by the RAB5A gene.
Ras-related protein Rab-7a is a protein that in humans is encoded by the RAB7A gene.

Ras-related protein Rab-11A is a protein that in humans is encoded by the RAB11A gene.
Ras-related protein Rab-4A is a protein that in humans is encoded by the RAB4A gene.

Reticulon 4 receptor (RTN4R) also known as Nogo-66 Receptor (NgR) or Nogo receptor 1 is a protein which in humans is encoded by the RTN4R gene. This gene encodes the receptor for reticulon 4, oligodendrocytemyelin glycoprotein and myelin-associated glycoprotein. This receptor mediates axonal growth inhibition and may play a role in regulating axonal regeneration and plasticity in the adult central nervous system.

RhoG is a small monomeric GTP-binding protein, and is an important component of many intracellular signalling pathways. It is a member of the Rac subfamily of the Rho family of small G proteins and is encoded by the gene RHOG.
Rab5 GDP/GTP exchange factor is a protein that in humans is encoded by the RABGEF1 gene.

Rab11 family-interacting protein 5 is a protein that in humans is encoded by the RAB11FIP5 gene.

Rab GTPase-binding effector protein 1 is an enzyme that in humans is encoded by the RABEP1 gene. It belongs to rabaptin protein family.
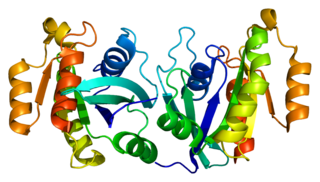
Ras-related protein Rab-11B is a protein that in humans is encoded by the RAB11B gene. Rab11b is reported as most abundantly expressed in brain, heart and testes.

Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.
The TBC (Tre-2/Bub2/Cdc16) is identified as a domain of some proteins or as a protein motif and widely recognized as a conserved one that includes approximately 200 amino acids in all eukaryotes.
Eps15 homology domain-containing protein 3, abbreviated as EDH3 and also known as PAST3, is a protein encoded by the EHD3 gene. It has been observed in humans, mice and rats. It belongs to the EHD protein family, a group of four membrane remodeling proteins related to the Dynamin superfamily of large GTPases. Although the four of them are 70-80% amino acid identical, they all have different locations. Its main function is related to endocytic transport.

Jean Gruenberg is a Swiss biologist, and a professor at the University of Geneva. His research in the fields of cell biology and biochemistry has significantly contributed to a better understanding of the molecular mechanisms involved in the intracellular traffic within eukaryotic cells, more especially in the endolysosomal pathway.
















