
Sedoreoviridae is a family of double-stranded RNA viruses. Member viruses have a wide host range, including vertebrates, invertebrates, plants, protists and fungi. They lack lipid envelopes and package their segmented genome within multi-layered capsids. Lack of a lipid envelope has allowed three-dimensional structures of these large complex viruses to be obtained, revealing a structural and likely evolutionary relationship to the cystovirus family of bacteriophage. There are currently 97 species in this family, divided among 15 genera in two subfamilies. Reoviruses can affect the gastrointestinal system and respiratory tract. The name "reo-" is an acronym for "respiratory enteric orphan" viruses. The term "orphan virus" refers to the fact that some of these viruses have been observed not associated with any known disease. Even though viruses in the family Reoviridae have more recently been identified with various diseases, the original name is still used.

Bunyavirales is an order of segmented negative-strand RNA viruses with mainly tripartite genomes. Member viruses infect arthropods, plants, protozoans, and vertebrates. It is the only order in the class Ellioviricetes. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.

Plant viruses are viruses that affect plants. Like all other viruses, plant viruses are obligate intracellular parasites that do not have the molecular machinery to replicate without a host. Plant viruses can be pathogenic to vascular plants.

Geminiviridae is a family of plant viruses that encode their genetic information on a circular genome of single-stranded (ss) DNA. There are 520 species in this family, assigned to 14 genera. Diseases associated with this family include: bright yellow mosaic, yellow mosaic, yellow mottle, leaf curling, stunting, streaks, reduced yields. They have single-stranded circular DNA genomes encoding genes that diverge in both directions from a virion strand origin of replication. According to the Baltimore classification they are considered class II viruses. It is the largest known family of single stranded DNA viruses.

Tick-borne encephalitis virus (TBEV) is a positive-strand RNA virus associated with tick-borne encephalitis in the genus Flavivirus.
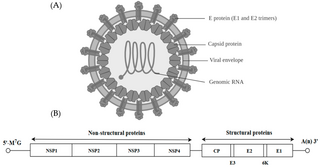
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 32 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.

Tomato bushy stunt virus (TBSV) is a virus of the tombusvirus family. It was first reported in tomatoes in 1935 and primarily affects vegetable crops, though it is not generally considered an economically significant plant pathogen. Depending upon the host, TBSV causes stunting of growth, leaf mottling, and deformed or absent fruit. The virus is likely to be soil-borne in the natural setting, but can also transmitted mechanically, for example through contaminated cutting tools. TBSV has been used as a model system in virology research on the life cycle of plant viruses, particularly in experimental infections of the model host plant Nicotiana benthamiana.

Orbivirus is a genus of double-stranded RNA viruses in the family Reoviridae and subfamily Sedoreovirinae. Unlike other reoviruses, orbiviruses are arboviruses. They can infect and replicate within a wide range of arthropod and vertebrate hosts. Orbiviruses are named after their characteristic doughnut-shaped capsomers.
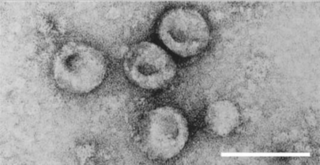
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.
Tenuivirus is a plant virus genus belonging to Phenuiviridae family in the order Bunyavirales. These plant viruses cause diseases in their host plants. Typical symptoms are chlorotic stripes on the affected leaves. This group of viruses make viral inclusions in infected cells which can be used to diagnose infection.

A viroplasm, sometimes called "virus factory" or "virus inclusion", is an inclusion body in a cell where viral replication and assembly occurs. They may be thought of as viral factories in the cell. There are many viroplasms in one infected cell, where they appear dense to electron microscopy. Very little is understood about the mechanism of viroplasm formation.
Rice stripe tenuivirus is an RNA plant pathogen of the genus Tenuivirus. It is prevalent in Japan, China, and Korea and can infect plants of the family Poaceae, which include wheat and corn. Damage from this disease causes major reductions in rice crop yield every year.
Yizhi Jane Tao is a Chinese biochemist, structural biologist, and Professor of Biochemistry and Cell Biology at Rice University in Houston, Texas. Professor Tao led a team of researchers to be the first to map the structure of the influenza A virus nucleoprotein to an atomic level, a feat which circulated widely in the popular press. She was named among the top ten most influential Chinese of 2006 by a consortium of China's leading media outlets including Phoenix Satellite Television, China News Service, Asia Newsweek, and World Journal.
Epizootic hemorrhagic disease virus, often abbreviated to EHDV, is a species of the genus Orbivirus, a member of the family Reoviridae. It is the causative agent of epizootic hemorrhagic disease, an acute, infectious, and often fatal disease of wild ruminants. In North America, the most severely affected ruminant is the white-tailed deer, although it may also infect mule deer, black-tailed deer, elk, bighorn sheep, and pronghorn antelope. It is often mistakenly referred to as “bluetongue virus” (BTV), another Orbivirus that like EHDV causes the host to develop a characteristic blue tongue due to systemic hemorrhaging and lack of oxygen in the blood. Despite showing clinical similarities, these two viruses are genetically distinct.
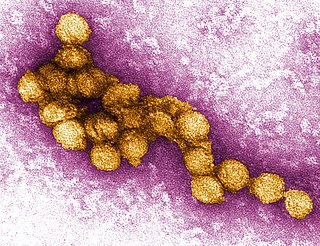
West Nile virus (WNV) is a single-stranded RNA virus that causes West Nile fever. It is a member of the family Flaviviridae, from the genus Flavivirus, which also contains the Zika virus, dengue virus, and yellow fever virus. The virus is primarily transmitted by mosquitoes, mostly species of Culex. The primary hosts of WNV are birds, so that the virus remains within a "bird–mosquito–bird" transmission cycle. The virus is genetically related to the Japanese encephalitis family of viruses.
Triatoma virus (TrV) is a virus belonging to the insect virus family Dicistroviridae. Within this family, there are currently 3 genera and 15 species of virus. Triatoma virus belongs to the genus Cripavirus. It is non-enveloped and its genetic material is positive-sense, single-stranded RNA. The natural hosts of triatoma virus are invertebrates. TrV is a known pathogen to Triatoma infestans, the major vector of Chagas disease in Argentina which makes triatoma virus a major candidate for biological vector control as opposed to chemical insecticides. Triatoma virus was first discovered in 1984 when a survey of pathogens of triatomes was conducted in the hopes of finding potential biological control methods for T. infestans.
Yokose virus (YOKV) is in the genus Flavivirus of the family Flaviviridae. Flaviviridae are often found in arthropods, such as mosquitoes and ticks, and may also infect humans. The genus Flavivirus includes over 50 known viruses, including Yellow Fever, West Nile Virus, Zika Virus, and Japanese Encephalitis. Yokose virus is a new member of the Flavivirus family that has only been identified in a few bat species. Bats have been associated with several emerging zoonotic diseases such as Ebola and SARS.
Sepik virus (SEPV) is an arthropod-borne virus (arbovirus) of the genus Flavivirus and family Flaviviridae. Flaviviridae is one of the most well characterized viral families, as it contains many well-known viruses that cause diseases that have become very prevalent in the world, like Dengue virus. The genus Flavivirus is one of the largest viral genera and encompasses over 50 viral species, including tick and mosquito borne viruses like Yellow fever virus and West Nile virus. Sepik virus is much less well known and has not been as well-classified as other viruses because it has not been known of for very long. Sepik virus was first isolated in 1966 from the mosquito Mansoniaseptempunctata, and it derives its name from the Sepik River area in Papua New Guinea, where it was first found. The geographic range of Sepik virus is limited to Papua New Guinea, due to its isolation.

Modoc virus (MODV) is a rodent-associated flavivirus. Small and enveloped, MODV contains positive single-stranded RNA. Taxonomically, MODV is part of the Flavivirus genus and Flaviviridae family. The Flavivirus genus includes nearly 80 viruses, both vector-borne and no known vector (NKV) species. Known flavivirus vector-borne viruses include Dengue virus, Yellow Fever virus, tick-borne encephalitis virus, and West Nile virus.
Rio Negro virus is an alphavirus that was first isolated in Argentina in 1980. The virus was first called Ag80-663 but was renamed to Rio Negro virus in 2005. It is a former member of the Venezuelan equine encephalitis complex (VEEC), which are a group of alphaviruses in the Americas that have the potential to emerge and cause disease. Río Negro virus was recently reclassified as a distinct species. Closely related viruses include Mucambo virus and Everglades virus.












