
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.

Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.

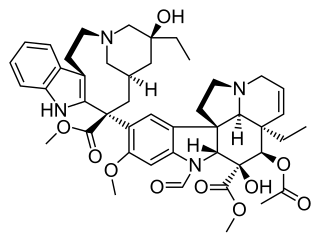
18-Methoxycoronaridine (18-MC) is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. 18-MC is a α3β4 nicotinic antagonist and, in contrast to ibogaine, has no affinity at the α4β2 subtype nor at NMDA-channels nor at the serotonin transporter, and has significantly reduced affinity for sodium channels and for the σ receptor, but retains modest affinity for μ-opioid receptors where it acts as an agonist, and κ-opioid receptors. The sites of action in the brain include the medial habenula, interpeduncular nucleus, dorsolateral tegmentum and basolateral amygdala. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.
Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in Lupinus mutabilis, and is thought to chelate the bivalent cations calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughan Williams classification of antiarrhythmic drugs.
Solenopsin is an alkaloid with the molecular formula C17H35N found in the venom of fire ants (Solenopsis). It is considered the primary toxin in the venom and may be the component responsible for the cardiorespiratory failure in people who experience excessive fire ant stings.

Sir Alan Rushton Battersby was an English organic chemist best known for his work to define the chemical intermediates in the biosynthetic pathway to vitamin B12 and the reaction mechanisms of the enzymes involved. His research group was also notable for its synthesis of radiolabelled precursors to study alkaloid biosynthesis and the stereochemistry of enzymic reactions. He won numerous awards including the Royal Medal in 1984 and the Copley Medal in 2000. He was knighted in the 1992 New Year Honours. Battersby died in February 2018 at the age of 92.

Glaucine is an aporphine alkaloid found in several different plant species in the family Papaveraceae such as Glaucium flavum, Glaucium oxylobum and Corydalis yanhusuo, and in other plants like Croton lechleri in the family Euphorbiaceae.

Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It has anti-inflammatory, immunologic and antiallergenic effects. It inhibits the degranulation of mast cells. It has a quinidine-like anti-arrhythmic effect. It is isolated from the plant Stephania tetrandra, and other Chinese and Japanese herbs. It has vasodilatory properties and can therefore reduce blood pressure. Tetrandrine may have potential use for the treatment of liver disease and liver cancer. Tetrandrine has potential therapeutic value to prevent excess scarring/fibrosis in conjunctiva following trabeculectomy or in patients with severe conjunctival inflammation. Tetrandrine has anti-inflammatory and anti-fibrogenic actions, which make tetrandrine and related compounds potentially useful in the treatment of lung silicosis, liver cirrhosis, and rheumatoid arthritis. Tetrandrine has also been shown to inhibit entry of Ebola virus into host cells in vitro and showed therapeutic efficacy against Ebola in preliminary studies on mice.

Dauricine is a plant metabolite, chemically classified as a phenol, an aromatic ether, and an isoquinoline alkaloid. It has been isolated from the Asian vine Menispermum dauricum, commonly known as Asian moonseed, and the North American vine Menispermum canadense, commonly known as Canadian moonseed. Scientists Tetsuji Kametani and Keiichiro Fukumoto of Japan are credited with being the first to synthesize dauricine in 1964, using both the Arndt-Eistert reaction and Bischler-Napieralski reaction to do so. Dauricine has been studied in vitro for its potential to inhibit cancer cell growth and to block cardiac transmembrane Na+, K+, and Ca2+ ion currents.

Solanidine is a poisonous steroidal alkaloid chemical compound that occurs in plants of the family Solanaceae, such as potato and Solanum americanum. Human ingestion of solanidine also occurs via the consumption of the glycoalkaloids, α-solanine and α-chaconine, present in potatoes. The sugar portion of these glycoalkaloids hydrolyses in the body, leaving the solanidine portion. Solanidine occurs in the blood serum of normal healthy people who eat potato, and serum solanidine levels fall markedly once potato consumption ceases. Solanidine from food is also stored in the human body for prolonged periods of time, and it has been suggested that it could be released during times of metabolic stress with the potential for deleterious consequences. Solanidine is responsible for neuromuscular syndromes via cholinesterase inhibition.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Gigactonine is a naturally occurring diterpene alkaloid first isolated from Aconitum gigas. It occurs widely in the Ranunculaceae plant family. The polycyclic ring system of this chemical compound contains nineteen carbon atoms and one nitrogen atom, which is the same as in aconitine and this is reflected in its preferred IUPAC name.

Cycleanine is a selective vascular calcium antagonist isolated from Stephania.

Erysodienone is a key precursor in the biosynthesis of many Erythrina-produced alkaloids. Early work was done by Derek Barton and co-workers to illustrate the biosynthetic pathways towards erythrina alkaloids. It was demonstrated that erysodienone could be synthesized from simple starting materials by a similar approach as its biosynthetic pathway, which led to the development of the biomimetic synthesis of erysodienone.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Dregamine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Ervatamia hirta and Tabernaemontana divaricata.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.


















