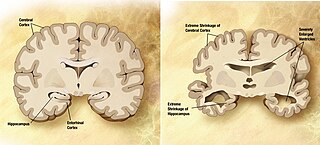
Career
At the start of his career in 1980, Tanzi worked as a research technologist for James Gusella at Massachusetts General Hospital. There, he assisted in localizing the Huntington's disease gene; his findings were published in Nature in 1983.
In 1987, based on his doctoral studies at Harvard Medical School, he was the lead author of seven papers published in Science and Nature between 1987 and 1988, describing the initial cloning, mapping, and characterization of the gene encoding the amyloid beta-protein precursor (APP). Two other groups reported the cloning of APP at that time, and the gene was shown in 1990 to contain a mutation causing Dutch cerebral hemorrhage with amyloidosis and later in 1991, a mutation causing early-onset familial AD (EO-FAD). In 1992, Tanzi and ex-trainee, Wilma Wasco, discovered the two APP family members, APLP1 and APLP2.
In 1995, Tanzi collaborated with Peter Hyslop and Jerry Schellenberg to discover the two other EO-FAD genes, presenilin 1 and 2 (PSEN1 and PSEN2). He has published many key studies characterizing the role of the EO-FAD genes in health and disease. All three genes remain among the most highly studied drug targets in the field of AD, especially about therapeutic strategies aimed at reducing beta-amyloid deposition. In 1993, Tanzi also first discovered the gene for the neurodegenerative disease, Wilson's disease; his findings were published in Nature Genetics . In that same year, he contributed to the discovery of the first familial amyotrophic lateral sclerosis (ALS) gene, SOD1, by providing the key genetic and physical mapping data for chromosome 21 used to find the gene defect.
As the leader of the Cure Alzheimer's Fund's Alzheimer's Genome Project, Tanzi several other AD genes, most notably, CD33, reported in 2008 with his ex-trainee, Lars Bertram, in the American Journal of Human Genetics . In that study, Tanzi reported the first family-based genome-wide association study of AD, which most notably to the identification of the first innate immune AD gene, CD33, which encodes a cell-surface receptor on monocytes and microglia. When first identified as an AD gene, nothing was known about CD33 in the brain or AD pathology. In 2013, Tanzi and his ex-trainee, Ana Griciuc first reported in Neuron that increased expression of CD33 in microglial cells in AD brain and showed that a protective CD33 gene variant was associated with reductions in CD33 expression and Abeta levels in AD brain. Importantly, they showed CD33 inhibits microglial phagocytosis and clearance of Abeta and induces pro-inflammatory cytokine release leading to neuroinflammation. They also elucidated the molecular mechanism by which sialic acid binds to CD33 to induce neuroinflammation.
In a follow-up study published in Neuron in 2019, Tanzi and Griciuc compared the neuroinflammatory effects of the CD33 gene to another AD-associated innate immune gene, TREM2. Knockout of CD33 in AD mice attenuated amyloid-beta pathology and improved cognition while knockout of TREM2 led to opposite effects. They then showed that TREM2 functions downstream of CD33 and that crosstalk between CD33 and TREM2 involves the neuroinflammation-related IL-1beta/IL-1RN axis cluster. CD33 has now emerged as the primary target for novel drug discovery programs aimed at curbing neuroinflammation, at over a dozen pharmaceutical and biotech companies.
Other AD genes Tanzi has discovered include ADAM10, UBQLN1, IDE, A2M, ITGB3, and ATXN1. In 2019, Tanzi, his ex-trainee, Jaehong Suh, and Huda Zoghbi (Baylor) led a study published in the journal, Cell , showing that ATXN1 (encoding the spinal cerebellar ataxia gene product, Ataxin-1) controls the production of the amyloid beta protein by regulating the expression of the gene BACE1.
Over the past two decades, Tanzi has also contributed to the development of novel therapeutics for AD. Beginning in 1994, in a study published in Science with his post-doctoral fellow Ashley Bush, Tanzi demonstrated a key role for zinc, copper, and iron in beta-amyloid deposition and Lewy body formation. This finding has led to the initiation of AD clinical trials of metal chaperones targeting metal-induced aggregation of beta-amyloid in AD and of alpha-synuclein and Lewy bodies in Parkinson's disease.
In 2000, Tanzi and Steven Wagner began screening for a class of drugs that they termed "gamma secretase modulators (GSM)". GSM's reverse the Abeta42:Abeta40 ratio and thereby prevent the “seeding” of amyloid plaques. Notably, they do not inhibit gamma-secretase. Tanzi and Wagner have published several papers on these compounds. Their ongoing drug development efforts supported by the National Institutes of Health Neurotherapeutics Blueprint Program and the Cure Alzheimer's Fund, have led to a clinical candidate GSM that is now slated for AD clinical trials.
Also in 2000, Tanzi collaborated with cell biologist, Dora Kovacs, to show that blocking the enzyme acetyl-coA acetyltransferase 1 (ACAT1), responsible for storing cholesterol as lipid droplets in intracellular rafts, prevents the generation of Abeta. Most recently, this led to their discovery that ACAT1 promotes the palmitoylation of APP dimers in lipid rafts, rendering them more susceptible to beta-secretase cleavage and Abeta production. They are now testing anti-palmitoylation drugs as well as their ACAT1 inhibitors as potential drugs for preventing the axonal release of Abeta and reducing beta-amyloid deposition.
In 2005, Tanzi and his ex-trainee and late colleague, Robert Moir, reported in the Journal of Biological Chemistry the existence of auto-antibodies against oligomeric Abeta, which they showed to protect against risk for AD. This discovery inspired Roger Nitsch and the Swiss biotech, Neurimmune to develop an AD therapy based on isolating those auto-antibodies from memory B-cells and reverse translating them into the promising beta-amyloid immunotherapy, known as aducanumab, which was recently successful in a phase 3 AD clinical trial by Biogen and Eisai.
In 2014, Tanzi, and his ex-trainees, Doo Yeon Kim and Se Hoon Choi, were the first to use human stem cells to create three-dimensional cell culture organoids of AD, dubbed by The New York Times as “Alzheimer's-in-a-Dish”. This model was the first to recapitulate all three key AD pathological hallmarks in vitro, and, most importantly, resolved a decades-long debate as to whether Abeta pathology causes the formation of neurofibrillary tangles. Using this system, they were the first to definitively show that amyloid plaques directly cause neurofibrillary tangles, something that could not be shown in mouse models of early-onset familial AD gene mutations in APP and the presenilins (owing to differences in mouse and human isoforms of the Tau protein, the principal component of neurofibrillary tangles). This 3-D cell culture model/human brain organoid system of AD has also made drug screening considerably faster and more cost-effective. Most recently, using a modified 3-D human stem cell-derived neural-glial cell AD model, Tanzi has helped develop therapies targeted against neuroinflammation in AD. These include ALZT-OP1 (AZTherapies) targeting microglial activation and neuroinflammation, and a neuroprotective drug combination, called AMX0035 (Amylyx, co-founded by Josh Cohen, Justin Klee with Tanzi serving as the founding chair of the Scientific Advisory Board). AMX0035 was successful in a phase 2 clinical trial of ALS and is now under consideration for approval by the FDA, while it is also being tested in a phase 2 clinical trial in AD patients.
In another set of groundbreaking studies, Tanzi, working with Robert Moir, investigated whether amyloid beta (Abeta) may play a normal role in the brain. They demonstrated Abeta to be a potent antimicrobial peptide (AMP) in the brain's innate immune system. After showing that the beta-amyloid protein protects against various infections in different animal models ranging from C. elegans to mouse models, they made an even more striking discovery. They showed that subclinical levels of microbes can rapidly seed (nucleate) amyloid plaques. It has long been held that amyloid plaques require a decade or more to form in the brain. However, injecting either bacteria or virus into the hippocampus of very young AD mice, showed that amyloid plaques formed overnight. These findings suggest that even subclinical levels of bacteria, viruses, or other microbes, entering or activating the brain, may initially trigger plaque formation and start the amyloid cascade rolling. Tanzi is currently carrying out large-scale metagenomic sequencing of post-mortem AD brains, to catalog the microbes that may be initiating amyloid pathology. In 2018, they published back-to-back papers with a group at Mt. Sinai implicating Herpes viruses in triggering plaque pathology in AD. Tanzi and Moir refer to this as the “antimicrobial protection hypothesis” of AD.
In other studies, Tanzi and his trainee, Zhongcong Xie, published several seminal papers providing the first evidence that the widely used general inhalant anesthetic, isoflurane, induces Abeta generation, apoptosis, and neurodegeneration in the mouse brain and post-operative CSF of patients. This has gradually led to a dramatic reduction in the clinical use of isoflurane in the operating room, especially in elderly patients and Alzheimer's patients. With trainee, Lee Goldstein, Tanzi showed how head injury due to a bomb blast or collision causes rapid induction of tangles and gliosis in mice. Now referred to as the “bobblehead” effect, it has been postulated to be the main cause of the subsequent onset of chronic traumatic encephalopathy in human subjects exposed to repeated concussion and head trauma.
Tanzi has testified to Congress on both Alzheimer's disease and most recently, in September 2019, on maintaining brain health. Tanzi serves on dozens of editorial and scientific advisory boards and as chair of the Cure Alzheimer's Fund Research Leadership Group. He has published over 600 research papers and has been issued numerous patents. He has co-authored three international bestsellers with Deepak Chopra. Tanzi has hosted three shows on public television: Super Brain with Rudy Tanzi, Super Genes with Tanzi, and The Brain, Body, Mind Connection. Tanzi regularly appears on network television programs, including CBS Morning News , The Today Show , NBC Nightly News , CNN, MSNBC, Oz, and Nova . [9]
Summary of key discoveries
- 1983: Helped localize the Huntington's disease gene via genetic linkage (with James F. Gusella and Anne Young).
- 1984-1988: Was among the first to discover the amyloid precursor protein (APP) gene and map it to chromosome 21, for which he produced the first complete linkage map.
- 1993: Carried out chromosome 21 physical mapping leading to first familial ALS gene-SOD1.
- 1993: Discovered the Wilson's disease gene.
- 1993: Carried out physical mapping of chromosome 21 to show SOD1 as the first gene causing familial amyotrophic lateral sclerosis with Bob Brown (U. Mass.).
- 1994: Showed zinc/copper drives Aβ aggregation and neurotoxicity.
- 1995: Cloned and discovered first mutations in AD gene presenilin 2; collaborated on the cloning of presenilin 1.
- 1999: Mapped the BACE2 gene to the obligate Down Syndrome region of chromosome 21
- 2000: Discovered genetic linkage of AD to chromosome 10, implicating the gene encoding the insulin-degrading enzyme.
- 2001: Showed that AD pathology in transgenic mice could be ameliorated by a zinc-copper chelator, clioquinol (PBT1)
- 2003: Clioquinol (PBT1) was successful in a phase 2 AD clinical trial.
- 2003: Discovered that apoptosis and caspase activation induces beta-amyloid deposition.
- 2005: Discovered ubiquitin 1 to be an AD gene.
- 2005: Showed auto-antibodies to oligomeric Aβ protect against AD – acknowledged to have significantly influenced the promising AD immunotherapy, Aducanumab.
- 2007: Established widely used gene databases: AlzGene, PDGene and SZGene.
- 2008: Employed family-based GWAS to discover the AD gene, CD33, the first AD gene controlling neuroinflammation and innate immunity in the brain, now a major drug target for AD.
- 2008: Showed first evidence that isoflurane (a general anesthetic) induces Aβ generation and neurodegeneration, leading to a dramatic reduction in its clinical use.
- 2010: Zinc-copper chelator, PBT2 (from Prana Biotechnology, now Alterity Therapeutics) successful in a phase 2 AD clinical trial.
- 2010: Discovered the first non-NSAID class of gamma secretase modulators (GSM), which selectively lower production of Aβ42 without off-target effects of gamma secretase inhibitors. A clinical candidate is slated for phase I clinical trials in 2021.
- 2010: Discovered and validated the first highly penetrant late-onset AD mutations in ADAM10, the main alpha-secretase that precludes Aβ production in the brain.
- 2010: Demonstrated Aβ to be a potent antimicrobial peptide in the brain's innate immune system.
- 2012: With Lee Goldstein, first showed that the “bobble head” effect of head injury/concussion causes tangle and gliosis pathology leading to chronic traumatic encephalopathy.
- 2013: Showed that CD33 controls neuroinflammation in AD at the microglial level.
- 2014: Invented a 3D human stem cell-derived neural culture AD model that recapitulated for the first time, plaques and tangles in vitro. This was the first model to definitively show that ?? amyloid plaques induce bona fide neurofibrillary tangles from endogenous tau.
- 2016: Demonstrated Aβ to be a potent antimicrobial peptide in the brain; showed for the first time in mice and 3D models that microbes can rapidly (overnight) seed deposition of β-amyloid as a defense mechanism of the brain's innate immune system.
- 2018: Invented a new 3D human stem cell-derived mixed neural-astrocyte-microglial microfluidic AD model, which showed neuronal Aβ deposition/tangle formation induces microglial activation and synaptic pruning/axotomy beginning with the astrocytic release of MCP1.
- 2018: Showed that the amyloid beta protein protects the brain against herpes virus infection.
- 2018: Demonstrated the key role of exercise-induced hippocampal neurogenesis in ameliorating AD pathology in AD transgenic mice and successfully mimicked this effect pharmacologically and genetically.
- 2019: Demonstrated that ataxin-1, the gene causing spinal cerebellar ataxia, regulates the production of amyloid beta by controlling the expression of the BACE1 gene.
- 2020: Used multiple whole genome sequencing datasets for the first time to identify sex-specific genetic risk factors for AD (ZBTB7C, GRID1, RIOK3, MCPH1) as well as several novel Alzheimer's disease-associated rare variants in loci related to synaptic function and neuronal development (FNBP1L, SEL1L, LINC00298, PRKCH, C15ORF41, C2CD3, KIF2A, APC, LHX9, NALCN, CTNNA2, SYTL3, CLSTN2, DTNB, DLG2).
- 2021: Showed astrocytic interleukin-3 programs microglia and reduces neuroinflammation in Alzheimer's disease. Co-authored successful clinical trial in ALS leading to approval of Relyvrio (Amylyx).
- 2022: Used whole-genome sequencing to discover two new genes associated with Alzheimer's disease: DTNB and DLG2. Discovered that the plasma IL-12/IFN-γ axis predicts cognitive trajectories in cognitively unimpaired older adults.