Insulin resistance (IR) is a pathological condition in which cells in insulin-sensitive tissues in the body fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia.

Leptin, also known as obese protein, is a protein hormone predominantly made by adipocytes. Its primary role is likely to regulate long-term energy balance.

Tumor necrosis factor (TNF), formerly known as TNF-α, is a chemical messenger that mediates the immune system and induces inflammation. TNF is produced primarily by activated macrophages, and induces inflammation by binding to its target receptors on other cells. It is a member of the tumor necrosis factor superfamily, a family of transmembrane proteins that are immunocytokines, chemical messengers of the immune system. Excessive production of TNF plays a critical role in several inflammatory diseases, and TNF-blocking drugs are often employed to treat these diseases.

Adipose tissue is a loose connective tissue composed mostly of adipocytes. It also contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells such as adipose tissue macrophages. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body.

Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. In cell culture, adipocyte progenitors can also form osteoblasts, myocytes and other cell types.
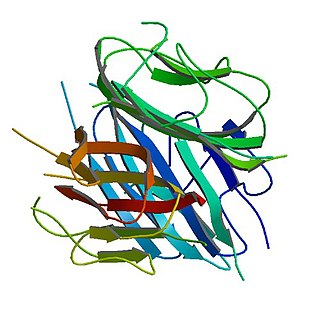
Adiponectin is a protein hormone and adipokine, which is involved in regulating glucose levels and fatty acid breakdown. In humans, it is encoded by the ADIPOQ gene and is produced primarily in adipose tissue, but also in muscle and even in the brain.

Resistin also known as adipose tissue-specific secretory factor (ADSF) or C/EBP-epsilon-regulated myeloid-specific secreted cysteine-rich protein (XCP1) is a cysteine-rich peptide hormone derived from adipose tissue that in humans is encoded by the RETN gene.
The adipokines, or adipocytokines are cytokines secreted by adipose tissue. Some contribute to an obesity-related low-grade state of inflammation or to the development of metabolic syndrome, a constellation of diseases including, but not limited to, type 2 diabetes, cardiovascular disease and atherosclerosis. The first adipokine to be discovered was leptin in 1994. Since that time, hundreds of adipokines have been discovered.
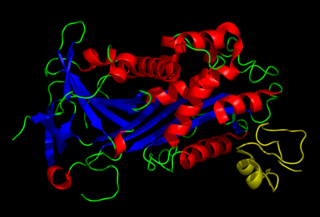
Plasminogen activator inhibitor-1 (PAI-1) also known as endothelial plasminogen activator inhibitor is a protein that in humans is encoded by the SERPINE1 gene. Elevated PAI-1 is a risk factor for thrombosis and atherosclerosis.

The chemokine ligand 2 (CCL2) is also referred to as monocyte chemoattractant protein 1 (MCP1) and small inducible cytokine A2. CCL2 is a small cytokine that belongs to the CC chemokine family. CCL2 tightly regulates cellular mechanics and thereby recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation produced by either tissue injury or infection.

Glucose transporter type 4 (GLUT4), also known as solute carrier family 2, facilitated glucose transporter member 4, is a protein encoded, in humans, by the SLC2A4 gene. GLUT4 is the insulin-regulated glucose transporter found primarily in adipose tissues and striated muscle. The first evidence for this distinct glucose transport protein was provided by David James in 1988. The gene that encodes GLUT4 was cloned and mapped in 1989.

Free Fatty acid receptor 4 (FFAR4), also termed G-protein coupled receptor 120 (GPR120), is a protein that in humans is encoded by the FFAR4 gene. This gene is located on the long arm of chromosome 10 at position 23.33. G protein-coupled receptors reside on their parent cells' surface membranes, bind any one of the specific set of ligands that they recognize, and thereby are activated to trigger certain responses in their parent cells. FFAR4 is a rhodopsin-like GPR in the broad family of GPRs which in humans are encoded by more than 800 different genes. It is also a member of a small family of structurally and functionally related GPRs that include at least three other free fatty acid receptors (FFARs) viz., FFAR1, FFAR2, and FFAR3. These four FFARs bind and thereby are activated by certain fatty acids.

alpha-2-HS-glycoprotein also known as fetuin-A is a protein that in humans is encoded by the AHSG gene. Fetuin-A belongs to the fetuin class of plasma binding proteins and is more abundant in fetal than adult blood.

Chemerin, also known as retinoic acid receptor responder protein 2 (RARRES2), tazarotene-induced gene 2 protein (TIG2), or RAR-responsive protein TIG2 is a protein that in humans is encoded by the RARRES2 gene.

Forkhead box protein O1 (FOXO1), also known as forkhead in rhabdomyosarcoma (FKHR), is a protein that in humans is encoded by the FOXO1 gene. FOXO1 is a transcription factor that plays important roles in regulation of gluconeogenesis and glycogenolysis by insulin signaling, and is also central to the decision for a preadipocyte to commit to adipogenesis. It is primarily regulated through phosphorylation on multiple residues; its transcriptional activity is dependent on its phosphorylation state.
A number of lifestyle factors are known to be important to the development of type 2 diabetes including: obesity, physical activity, diet, stress, and urbanization. Excess body fat underlies 64% of cases of diabetes in men and 77% of cases in women. A number of dietary factors such as sugar sweetened drinks and the type of fat in the diet appear to play a role.
In recent years it has become apparent that the environment and underlying mechanisms affect gene expression and the genome outside of the central dogma of biology. It has been found that many epigenetic mechanisms are involved in the regulation and expression of genes such as DNA methylation and chromatin remodeling. These epigenetic mechanisms are believed to be a contributing factor to pathological diseases such as type 2 diabetes. An understanding of the epigenome of diabetes patients may help to elucidate otherwise hidden causes of this disease.
Asprosin is a protein hormone produced by mammals in tissues that stimulates the liver to release glucose into the blood stream. Asprosin is encoded by the gene FBN1 as part of the protein profibrillin and is released from the C-terminus of the latter by specific proteolysis. In the liver, asprosin activates rapid glucose release via a cyclic adenosine monophosphate (cAMP)-dependent pathway.

Pathophysiology of obesity is the study of disordered physiological processes that cause, result from, or are otherwise associated with obesity. A number of possible pathophysiological mechanisms have been identified which may contribute in the development and maintenance of obesity.
Hepatokines are proteins produced by liver cells (hepatocytes) that are secreted into the circulation and function as hormones across the organism. Research is mostly focused on hepatokines that play a role in the regulation of metabolic diseases such as diabetes and fatty liver and include: Adropin, ANGPTL4, Fetuin-A, Fetuin-B, FGF-21, Hepassocin, LECT2, RBP4,Selenoprotein P, Sex hormone-binding globulin.
















