Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number of compounds in a single process. These compound libraries can be made as mixtures, sets of individual compounds or chemical structures generated by computer software. Combinatorial chemistry can be used for the synthesis of small molecules and for peptides.

In an aqueous solution, precipitation is the "sedimentation of a solid material from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix normally in the form of small microbeads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a large surface area on and inside them where the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin, that differ in composition if the target is an anion or a cation. Most commercial resins are made of polystyrene sulfonate, followed up by polyacrylate.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with normal synthesis in a liquid state include:

Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of chemicals, and separation of substances.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.
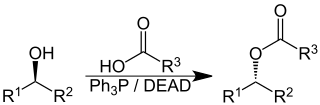
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). In a typical protocol, one dissolves the alcohol, the carboxylic acid, and triphenylphosphine in tetrahydrofuran or other suitable solvent, cool to 0 °C using an ice-bath, slowly add the DEAD dissolved in THF, then stir at room temperature for several hours. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion as the nucleophile displaces it. A common side-product is produced when the azodicarboxylate displaces the leaving group instead of the desired nucleophile. This happens if the nucleophile is not acidic enough or is not nucleophilic enough due to steric or electronic constraints. A variation of this reaction utilizing a nitrogen nucleophile is known as a Fukuyama–Mitsunobu.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.

Merrifield Resin is a cross-linked polystyrene resin that carries a chloromethyl functional group. Merrifield resin is named after its inventor, Robert Bruce Merrifield, and used in solid-phase synthesis. The material is typically available as white beads. These beads are allowed to swell in suitable solvents, which then allows the reagents to substitute the chloride substituents.
Acid–base extraction is a subclass of liquid–liquid extractions and involves the separation of chemical species from other acidic or basic compounds. It is typically performed during the work-up step following a chemical synthesis to purify crude compounds and results in the product being largely free of acidic or basic impurities. A separatory funnel is commonly used to perform an acid-base extraction.
In chemistry, work-up refers to the series of manipulations required to isolate and purify the product(s) of a chemical reaction. The term is used colloquially to refer to these manipulations, which may include:

An immobilized enzyme is an enzyme, with restricted mobility, attached to an inert, insoluble material—such as calcium alginate. This can provide increased resistance to changes in conditions such as pH or temperature. It also lets enzymes be held in place throughout the reaction, following which they are easily separated from the products and may be used again - a far more efficient process and so is widely used in industry for enzyme catalysed reactions. An alternative to enzyme immobilization is whole cell immobilization. Immobilized enzymes are easily to be handled, simply separated from their products, and can be reused.

Methyltrichlorosilane, also known as trichloromethylsilane, is a monomer and organosilicon compound with the formula CH3SiCl3. It is a colorless liquid with a sharp odor similar to that of hydrochloric acid. As methyltrichlorosilane is a reactive compound, it is mainly used a precursor for forming various cross-linked siloxane polymers.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.
The Pinnick oxidation is an organic reaction by which aldehydes can be oxidized into their corresponding carboxylic acids using sodium chlorite (NaClO2) under mild acidic conditions. It was originally developed by Lindgren and Nilsson. The typical reaction conditions used today were developed by G. A. Kraus. H.W. Pinnick later demonstrated that these conditions could be applied to oxidize α,β-unsaturated aldehydes. There exist many different reactions to oxidize aldehydes, but only a few are amenable to a broad range of functional groups. The Pinnick oxidation has proven to be both tolerant of sensitive functionalities and capable of reacting with sterically hindered groups. This reaction is especially useful for oxidizing α,β-unsaturated aldehydes, and another one of its advantages is its relatively low cost.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).
The split and pool (split-mix) synthesis is a method in combinatorial chemistry that can be used to prepare combinatorial compound libraries. It is a stepwise, highly efficient process realized in repeated cycles. The procedure makes it possible to prepare millions or even trillions of compounds as mixtures that can be used in drug research.











