Apiaceae or Umbelliferae is a family of mostly aromatic flowering plants named after the type genus Apium and commonly known as the celery, carrot or parsley family, or simply as umbellifers. It is the 16th-largest family of flowering plants, with more than 3,700 species in 434 genera including such well-known and economically important plants as ajwain, angelica, anise, asafoetida, caraway, carrot, celery, chervil, coriander, cumin, dill, fennel, lovage, cow parsley, parsley, parsnip and sea holly, as well as silphium, a plant whose identity is unclear and which may be extinct.

Celery is a marshland plant in the family Apiaceae that has been cultivated as a vegetable since antiquity. Celery has a long fibrous stalk tapering into leaves. Depending on location and cultivar, either its stalks, leaves or hypocotyl are eaten and used in cooking. Celery seed powder is used as a spice.

Parsley, or garden parsley is a species of flowering plant in the family Apiaceae that is native to the central and eastern Mediterranean region, but has been naturalized elsewhere in Europe, and is widely cultivated as a herb, and a vegetable.

Aioli, allioli or aïoli is a cold sauce consisting of an emulsion of garlic, salt, olive oil, and often egg; it is found in the cuisines of the northwest Mediterranean, from Andalusia to Calabria.

Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."

Asafoetida is the dried latex exuded from the rhizome or tap root of several species of Ferula, perennial herbs growing 1 to 1.5 m tall. They are part of the celery family, Umbelliferae. Asafoetida is thought to be in the same genus as silphium, a North African plant now believed to be extinct, and was used as a cheaper substitute for that historically important herb from classical antiquity. The species are native to the deserts of Iran and mountains of Afghanistan where substantial amounts are grown.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

Myristicin is a naturally occurring compound found in common herbs and spices, the most well known being nutmeg. It is an insecticide, and has been shown to enhance the effectiveness of other insecticides in combination. Myristicin is also a precursor for substituted amphetamine derivative compounds structurally related to MMDA and MDMA; it is believed to be metabolized in the liver into MMDA, but unlikely since no MMDA was found in urine, in the body it produces hallucinogenic effects, and can be converted to MMDMA in controlled chemical synthesis. It interacts with many enzymes and signaling pathways in the body, is cytotoxic to living cells, and may also have chemoprotective properties.

Phyllocladus, the celery pines, is a small genus of conifers, now usually treated in the family Podocarpaceae.Species occur mainly in New Zealand, Tasmania, and Malesia in the Southern Hemisphere, though P. hypophyllus ranges into the Philippines, a short way north of the equator.

An insect repellent is a substance applied to skin, clothing, or other surfaces to discourage insects from landing or climbing on that surface. Insect repellents help prevent and control the outbreak of insect-borne diseases such as malaria, Lyme disease, dengue fever, bubonic plague, river blindness, and West Nile fever. Pest animals commonly serving as vectors for disease include insects such as flea, fly, and mosquito; and ticks (arachnids).
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

Pickling is the process of preserving or extending the shelf life of food by either anaerobic fermentation in brine or immersion in vinegar. The pickling procedure typically affects the food's texture and flavor. The resulting food is called a pickle, or, to prevent ambiguity, prefaced with pickled. Foods that are pickled include vegetables, fruits, meats, fish, dairy and eggs.

Smyrnium olusatrum, common name alexanders is an edible flowering plant of the family Apiaceae (Umbelliferae), which grows on waste ground and in hedges around the Mediterranean and Atlantic coastal regions of Europe. It was formerly widely grown as a pot herb, but is now appreciated mostly by foragers.

In chemistry, a polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)
n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (−CR=CR′−). They are also sometimes referred to as oligoynes, or carbinoids after "carbyne" (−C≡C−)
∞, the hypothetical allotrope of carbon that would be the ultimate member of the series. The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today.
Guaiacol is a naturally-occurring organic compound with the formula C6H4(OH)(OCH3). Although it is biosynthesized by a variety of organisms, this aromatic oil is usually derived from guaiacum or wood creosote. It is also found in essential oils from celery seeds, tobacco leaves, orange leaves, and lemon peels. The pure substance is colorless, but samples become yellow upon exposure to air and light. Guaiacol is present in wood smoke, resulting from the pyrolysis of lignin. The compound contributes to the flavor of many substances such as whisky and roasted coffee.

Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.

In botany, the petiole is the stalk that attaches the leaf blade to the stem, and is able to twist the leaf to face the sun. This gives a characteristic foliage arrangement to the plant. Outgrowths appearing on each side of the petiole in some species are called stipules. Leaves with a petiole are said to be petiolate, while leaves lacking a petiole are called sessile or apetiolate.
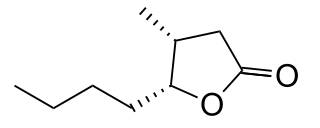
cis-3-Methyl-4-octanolide, also called cis-β-methyl-γ-octalactone or 5-butyldihydro-4-methylfuran-2(3H)-one, is a chemical compound of the lactone family with formula C
9H
16O
2. It exists in two optical isomers: 3R,4R ("+") and 3S,4S ("−").

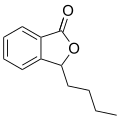
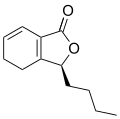
Butylphthalide is one of the chemical constituents in celery oil, along with sedanolide, which is primarily responsible for the aroma and taste of celery.

Ligusticum scoticum, known as Scots lovage, or Scottish licorice-root, is a perennial flowering plant in the celery family Apiaceae found near the coasts of northern Europe and north-eastern North America. It grows up to 60 centimetres (24 in) tall and is found in rock crevices and cliff-top grassland. It is closely related to, and possibly conspecific with, Ligusticum hultenii from the coast of the northern Pacific Ocean. The plant is edible and contains the compound sotolon, which is also present in fenugreek. The leaves have a flavour similar to parsley or celery, while the seeds taste similar to fenugreek or cumin.