
Down syndrome or Down's syndrome, also known as trisomy 21, is a genetic disorder caused by the presence of all or part of a third copy of chromosome 21. It is usually associated with developmental delays, mild to moderate intellectual disability, and characteristic physical features.

Amniocentesis is a medical procedure used primarily in the prenatal diagnosis of genetic conditions. It has other uses such as in the assessment of infection and fetal lung maturity. Prenatal diagnostic testing, which includes amniocentesis, is necessary to conclusively diagnose the majority of genetic disorders, with amniocentesis being the gold-standard procedure after 15 weeks' gestation.

Genetic testing, also known as DNA testing, is used to identify changes in DNA sequence or chromosome structure. Genetic testing can also include measuring the results of genetic changes, such as RNA analysis as an output of gene expression, or through biochemical analysis to measure specific protein output. In a medical setting, genetic testing can be used to diagnose or rule out suspected genetic disorders, predict risks for specific conditions, or gain information that can be used to customize medical treatments based on an individual's genetic makeup. Genetic testing can also be used to determine biological relatives, such as a child's biological parentage through DNA paternity testing, or be used to broadly predict an individual's ancestry. Genetic testing of plants and animals can be used for similar reasons as in humans, to gain information used for selective breeding, or for efforts to boost genetic diversity in endangered populations.

Prenatal testing is a tool that can be used to detect some birth defects at various stages prior to birth. Prenatal testing consists of prenatal screening and prenatal diagnosis, which are aspects of prenatal care that focus on detecting problems with the pregnancy as early as possible. These may be anatomic and physiologic problems with the health of the zygote, embryo, or fetus, either before gestation even starts or as early in gestation as practicable. Screening can detect problems such as neural tube defects, chromosome abnormalities, and gene mutations that would lead to genetic disorders and birth defects such as spina bifida, cleft palate, Down syndrome, trisomy 18, Tay–Sachs disease, sickle cell anemia, thalassemia, cystic fibrosis, muscular dystrophy, and fragile X syndrome. Some tests are designed to discover problems which primarily affect the health of the mother, such as PAPP-A to detect pre-eclampsia or glucose tolerance tests to diagnose gestational diabetes. Screening can also detect anatomical defects such as hydrocephalus, anencephaly, heart defects, and amniotic band syndrome.
The triple test, also called triple screen, the Kettering test or the Bart's test, is an investigation performed during pregnancy in the second trimester to classify a patient as either high-risk or low-risk for chromosomal abnormalities.
The Pallister–Killian syndrome (PKS), also termed tetrasomy 12p mosaicism or the Pallister mosaic aneuploidy syndrome, is an extremely rare and severe genetic disorder. PKS is due to the presence of an extra and abnormal chromosome termed a small supernumerary marker chromosome (sSMC). sSMCs contain copies of genetic material from parts of virtually any other chromosome and, depending on the genetic material they carry, can cause various genetic disorders and neoplasms. The sSMC in PKS consists of multiple copies of the short arm of chromosome 12. Consequently, the multiple copies of the genetic material in the sSMC plus the two copies of this genetic material in the two normal chromosome 12's are overexpressed and thereby cause the syndrome. Due to a form of genetic mosaicism, however, individuals with PKS differ in the tissue distributions of their sSMC and therefore show different syndrome-related birth defects and disease severities. For example, individuals with the sSMC in their heart tissue are likely to have cardiac structural abnormalities while those without this sSMC localization have a structurally normal heart.
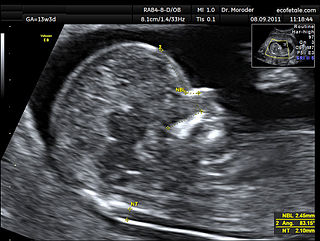
A nuchal scan or nuchal translucency (NT) scan/procedure is a sonographic prenatal screening scan (ultrasound) to detect chromosomal abnormalities in a fetus, though altered extracellular matrix composition and limited lymphatic drainage can also be detected.
Noninvasive genotyping is a modern technique for obtaining DNA for genotyping that is characterized by the indirect sampling of specimen, not requiring harm to, handling of, or even the presence of the organism of interest. Beginning in the early 1990s, with the advent of PCR, researchers have been able to obtain high-quality DNA samples from small quantities of hair, feathers, scales, or excrement. These noninvasive samples are an improvement over older allozyme and DNA sampling techniques that often required larger samples of tissue or the destruction of the studied organism. Noninvasive genotyping is widely utilized in conservation efforts, where capture and sampling may be difficult or disruptive to behavior. Additionally, in medicine, this technique is being applied in humans for the diagnosis of genetic disease and early detection of tumors. In this context, invasivity takes on a separate definition where noninvasive sampling also includes simple blood samples.
The genetics and abortion issue is an extension of the abortion debate and the disability rights movement. Since the advent of forms of prenatal diagnosis, such as amniocentesis and ultrasound, it has become possible to detect the presence of congenital disorders in the fetus before birth. Specifically, disability-selective abortion is the abortion of fetuses that are found to have non-fatal mental or physical defects detected through prenatal testing. Many prenatal tests are now considered routine, such as testing for Down syndrome. Women who are discovered to be carrying fetuses with disabilities are often faced with the decision of whether to abort or to prepare to parent a child with disabilities.
Dr. Paul R. Billings is a distinguished American doctor, lecturer, researcher, professor, and consultant on genetic information. His research interests include the impact of genomic data on society, the integration of genomics with diagnostics in health and medical care, and individualised genomic medicine. He is the author of over 250 publications and has appeared on talk shows such as The Oprah Winfrey Show and 60 Minutes. He is currently the CEO and Director of Biological Dynamics.
Ravgen Inc. is a privately owned biotech company founded in 2000 by Chairman and C.E.O. Dr. Ravinder Dhallan. Ravgen Inc. performs research in the prenatal diagnostic field and has developed non-invasive prenatal diagnosis testing for Down Syndrome.
Ravinder (Rav) Dhallan is the chairman and chief executive officer of Ravgen.
Natera, Inc. is a clinical genetic testing company based in Austin, Texas that specializes in non-invasive, cell-free DNA (cfDNA) testing technology, with a focus on women’s health, cancer, and organ health. Natera’s proprietary technology combines novel molecular biology techniques with a suite of bioinformatics software that allows detection down to a single molecule in a tube of blood. Natera operates CAP-accredited laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) in San Carlos, California and Austin, Texas.
Cell-free fetal DNA (cffDNA) is fetal DNA that circulates freely in the maternal blood. Maternal blood is sampled by venipuncture. Analysis of cffDNA is a method of non-invasive prenatal diagnosis frequently ordered for pregnant women of advanced age. Two hours after delivery, cffDNA is no longer detectable in maternal blood.

Molecular diagnostics is a collection of techniques used to analyze biological markers in the genome and proteome, and how their cells express their genes as proteins, applying molecular biology to medical testing. In medicine the technique is used to diagnose and monitor disease, detect risk, and decide which therapies will work best for individual patients, and in agricultural biosecurity similarly to monitor crop- and livestock disease, estimate risk, and decide what quarantine measures must be taken.

Dennis Lo Yuk-ming is a Hong Kong molecular biologist who has been serving as the vice-chancellor and president of the Chinese University of Hong Kong (CUHK) since 8 January 2025. His research focuses on the detection of cell-free fetal DNA in blood plasma, and he is best known for his contributions to the development of non-invasive prenatal testing

Diana W. Bianchi is the director of the U.S. National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development, a post often called “the nation’s pediatrician.” She is a medical geneticist and neonatologist noted for her research on fetal cell microchimerism and prenatal testing. Bianchi had previously been the Natalie V. Zucker Professor of Pediatrics, Obstetrics, and Gynecology at Tufts University School of Medicine and founder and executive director of the Mother Infant Research Institute at Tufts Medical Center. She also has served as Vice Chair for Research in the Department of Pediatrics at the Floating Hospital for Children at Tufts Medical Center.
Ariosa Diagnostics, Inc. v. Sequenom, Inc., 788 F.3d 1371, is a controversial decision of the US Federal Circuit in which the court applied the Mayo v. Prometheus test to invalidate on the basis of subject matter eligibility a patent said to "solve ... a very practical problem accessing fetal DNA without creating a major health risk for the unborn child." The rationale for denying patent-eligibility in this case allegedly stems from claims being directed toward non-eligible subject matter , "if the APPLICATION [of this discovery] merely relies upon elements already known in the art."
Noninvasive prenatal testing (NIPT) is a method used to determine the risk for the fetus being born with certain chromosomal abnormalities, such as trisomy 21, trisomy 18 and trisomy 13. This testing analyzes small DNA fragments that circulate in the blood of a pregnant woman. Unlike most DNA found in the nucleus of a cell, these fragments are not found within the cells, instead they are free-floating, and so are called cell free fetal DNA (cffDNA). These fragments usually contain less than 200 DNA building blocks and arise when cells die, and their contents, including DNA, are released into the bloodstream. CffDNA derives from placental cells and is usually identical to fetal DNA. Analysis of cffDNA from placenta provides the opportunity for early detection of certain chromosomal abnormalities without harming the fetus.
Dame Lyn Susan Chitty is a British physician and Professor of Genetics and Fetal Medicine at University College London. She is the deputy director of the National Institute for Health and Care Research Great Ormond Street Hospital Biomedical Research Centre. She is the 2022 president of the International Society for Prenatal Diagnosis. Her research considers non-invasive prenatal diagnostics. She was made a Dame in the 2022 New Year Honours.







