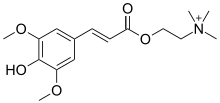
Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.

Rapeseed, also known as rape and oilseed rape, is a bright-yellow flowering member of the family Brassicaceae, cultivated mainly for its oil-rich seed, which naturally contains appreciable amounts of mildly toxic erucic acid. The term "canola" denotes a group of rapeseed cultivars that were bred to have very low levels of erucic acid and which are especially prized for use as human and animal food. Rapeseed is the third-largest source of vegetable oil and the second-largest source of protein meal in the world.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include phenolic acids, flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.
Gibberellins (GAs) are plant hormones that regulate various developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence. They are one of the longest-known classes of plant hormone. It is thought that the selective breeding of crop strains that were deficient in GA synthesis was one of the key drivers of the "green revolution" in the 1960s, a revolution that is credited to have saved over a billion lives worldwide.

Lecithin is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues which are amphiphilic – they attract both water and fatty substances, and are used for smoothing food textures, emulsifying, homogenizing liquid mixtures, and repelling sticking materials.

The yacón is a species of daisy traditionally grown in the northern and central Andes from Colombia to northern Argentina for its crisp, sweet-tasting, tuberous roots. Their texture and flavour are very similar to jícama, mainly differing in that yacón has some slightly sweet, resinous, and floral undertones to its flavour, probably due to the presence of inulin, which produces the sweet taste of the roots of elecampane, as well. Another name for yacón is Peruvian ground apple, possibly from the French name of potato, pomme de terre. The tuber is composed mostly of water and various polysaccharides.

Sinapinic acid, or sinapic acid (Sinapine - Origin: L. Sinapi, sinapis, mustard, Gr., cf. F. Sinapine.), is a small naturally occurring hydroxycinnamic acid. It is a member of the phenylpropanoid family. It is a commonly used matrix in MALDI mass spectrometry. It is a useful matrix for a wide variety of peptides and proteins. It serves well as a matrix for MALDI due to its ability to absorb laser radiation and to also donate protons (H+) to the analyte of interest.

p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.

Myrosinase is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB.
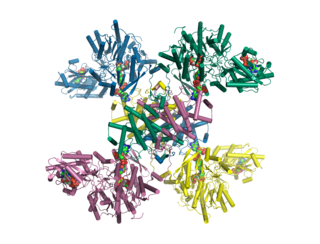
ATP citrate synthase (also ATP citrate lyase (ACLY)) is an enzyme that in animals catalyzes an important step in fatty acid biosynthesis. By converting citrate to acetyl-CoA, the enzyme links carbohydrate metabolism, which yields citrate as an intermediate, with fatty acid biosynthesis, which consumes acetyl-CoA. In plants, ATP citrate lyase generates cytosolic acetyl-CoA precursors of thousands of specialized metabolites, including waxes, sterols, and polyketides.
In enzymology, a sinapoylglucose---choline O-sinapoyltransferase is an enzyme that catalyzes the chemical reaction
The biosynthesis of phenylpropanoids involves a number of enzymes.
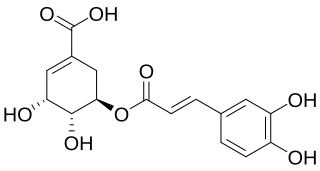
Dactylifric acid is an ester derived from caffeic acid and shikimic acid. It and its isomers are enzymic browning substrates found in dates.

Ambadi seed oil is extracted from seeds of the ambadi plant. It is an annual or perennial plant in the family Malvaceae and related to the roselle. It is believed to be native to Africa or Tropical Asia.
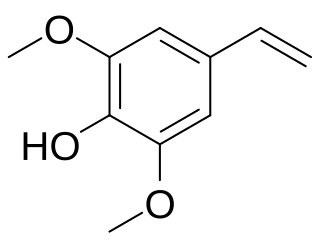
Canolol is a phenolic compound found in crude canola oil. It is produced by decarboxylation of sinapic acid during canola seed roasting.
Galactomyces reessii is a yeast, belonging to the genus Galactomyces. G. reessii that lives in soil and dissolves pectin.
Kalahari melon oil also known as Tsamma (Damara/Nama), wild watermelon (English), bitterboela, karkoer (Afrikaans), wild watermelon, makatane (Setswana) or Mokaté oil, is a plant oil, extracted from the seeds of the Kalahari melon (Citrullus vulgaris), which is endemic to the Kalahari Desert, spanning Namibia, Botswana and South Africa. Being one of 1,200 varieties of melon, Kalahari melon oil is distinct from regular watermelon seed oil. The seed of the Kalahari melon consists of approximately 50% oil, 35% protein and 5% dietary fibre.

Rapeseed oil is one of the oldest known vegetable oils. There are both edible and industrial forms produced from rapeseed, the seed of several cultivars of the plant family Brassicaceae. Historically, it was restricted as a food oil due to its content of erucic acid. Laboratory studies about this acid have shown damage to the cardiac muscle of laboratory animals in high quantities. It also imparts a bitter taste, and glucosinolates, which made many parts of the plant less nutritious in animal feed. Rapeseed oil from standard cultivars can contain up to 54% erucic acid.