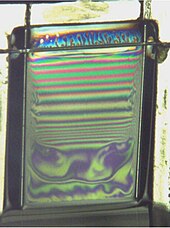
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects to float on a water surface without becoming even partly submerged.

Foams are two-phase material systems where a gas is dispersed in a second, non-gaseous material, specifically, in which gas cells are enclosed by a distinct liquid or solid material. The foam "may contain more or less liquid [or solid] according to circumstances", although in the case of gas-liquid foams, the gas occupies most of the volume. The word derives from the medieval German and otherwise obsolete veim, in reference to the "frothy head forming in the glass once the beer has been freshly poured".

A soap bubble is an extremely thin film of soap or detergent and water enclosing air that forms a hollow sphere with an iridescent surface. Soap bubbles usually last for only a few seconds before bursting, either on their own or on contact with another object. They are often used for children's enjoyment, but they are also used in artistic performances. Assembling many bubbles results in foam.

In surface science, surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energetically favorable than the bulk of the material, otherwise there would be a driving force for surfaces to be created, removing the bulk of the material by sublimation. The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is the work required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. There is "excess energy" as a result of the now-incomplete, unrealized bonding between the two created surfaces.
A thin film is a layer of materials ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films is a fundamental step in many applications. A familiar example is the household mirror, which typically has a thin metal coating on the back of a sheet of glass to form a reflective interface. The process of silvering was once commonly used to produce mirrors, while more recently the metal layer is deposited using techniques such as sputtering. Advances in thin film deposition techniques during the 20th century have enabled a wide range of technological breakthroughs in areas such as magnetic recording media, electronic semiconductor devices, integrated passive devices, light-emitting diodes, optical coatings, hard coatings on cutting tools, and for both energy generation and storage. It is also being applied to pharmaceuticals, via thin-film drug delivery. A stack of thin films is called a multilayer.

Wetting is the ability of a liquid to displace gas to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. There are two types of wetting: non-reactive wetting and reactive wetting.
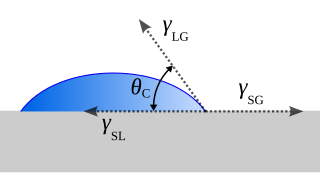
In fluid mechanics, dewetting is one of the processes that can occur at a solid–liquid, solid–solid or liquid–liquid interface. Generally, dewetting describes the process of retraction of a fluid from a non-wettable surface it was forced to cover. The opposite process—spreading of a liquid on a substrate—is called wetting. The factor determining the spontaneous spreading and dewetting for a drop of liquid placed on a solid substrate with ambient gas, is the so-called spreading coefficient S:

The Marangoni effect is the mass transfer along an interface between two phases due to a gradient of the surface tension. In the case of temperature dependence, this phenomenon may be called thermo-capillary convection.
A Rollin film, named after Bernard V. Rollin, is a 30 nm-thick liquid film of helium in the helium II state. It exhibits a "creeping" effect in response to surfaces extending past the film's level. Helium II can escape from any non-closed container via creeping toward and eventually evaporating from capillaries of 10 to 100 nm or greater.

A Langmuir–Blodgett (LB) film is an emerging kind of 2D materials to fabricate heterostructures for nanotechnology, formed when Langmuir films—or Langmuir monolayers (LM)—are transferred from the liquid-gas interface to solid supports during the vertical passage of the support through the monolayers. LB films can contain one or more monolayers of an organic material, deposited from the surface of a liquid onto a solid by immersing the solid substrate into the liquid. A monolayer is adsorbed homogeneously with each immersion or emersion step, thus films with very accurate thickness can be formed. This thickness is accurate because the thickness of each monolayer is known and can therefore be added to find the total thickness of a Langmuir–Blodgett film.

The phenomenon called tears of wine is manifested as a ring of clear liquid, near the top of a glass of wine, from which droplets continuously form and drop back into the wine. It is most readily observed in a wine which has a high alcohol content. It is also referred to as wine legs, fingers, curtains, church windows, or feet.

In physics, a "coffee ring" is a pattern left by a puddle of particle-laden liquid after it evaporates. The phenomenon is named for the characteristic ring-like deposit along the perimeter of a spill of coffee. It is also commonly seen after spilling red wine. The mechanism behind the formation of these and similar rings is known as the coffee ring effect or in some instances, the coffee stain effect, or simply ring stain.
The Marangoni number (Ma) is, as usually defined, the dimensionless number that compares the rate of transport due to Marangoni flows, with the rate of transport of diffusion. The Marangoni effect is flow of a liquid due to gradients in the surface tension of the liquid. Diffusion is of whatever is creating the gradient in the surface tension. Thus as the Marangoni number compares flow and diffusion timescales it is a type of Péclet number.

A Wilhelmy plate is a thin plate that is used to measure equilibrium surface or interfacial tension at an air–liquid or liquid–liquid interface. In this method, the plate is oriented perpendicular to the interface, and the force exerted on it is measured. Based on the work of Ludwig Wilhelmy, this method finds wide use in the preparation and monitoring of Langmuir films.

The capillary length or capillary constant is a length scaling factor that relates gravity and surface tension. It is a fundamental physical property that governs the behavior of menisci, and is found when body forces (gravity) and surface forces are in equilibrium.
Stranski–Krastanov growth is one of the three primary modes by which thin films grow epitaxially at a crystal surface or interface. Also known as 'layer-plus-island growth', the SK mode follows a two step process: initially, complete films of adsorbates, up to several monolayers thick, grow in a layer-by-layer fashion on a crystal substrate. Beyond a critical layer thickness, which depends on strain and the chemical potential of the deposited film, growth continues through the nucleation and coalescence of adsorbate 'islands'. This growth mechanism was first noted by Ivan Stranski and Lyubomir Krastanov in 1938. It wasn't until 1958 however, in a seminal work by Ernst Bauer published in Zeitschrift für Kristallographie, that the SK, Volmer–Weber, and Frank–van der Merwe mechanisms were systematically classified as the primary thin-film growth processes. Since then, SK growth has been the subject of intense investigation, not only to better understand the complex thermodynamics and kinetics at the core of thin-film formation, but also as a route to fabricating novel nanostructures for application in the microelectronics industry.

The vapor–liquid–solid method (VLS) is a mechanism for the growth of one-dimensional structures, such as nanowires, from chemical vapor deposition. The growth of a crystal through direct adsorption of a gas phase on to a solid surface is generally very slow. The VLS mechanism circumvents this by introducing a catalytic liquid alloy phase which can rapidly adsorb a vapor to supersaturation levels, and from which crystal growth can subsequently occur from nucleated seeds at the liquid–solid interface. The physical characteristics of nanowires grown in this manner depend, in a controllable way, upon the size and physical properties of the liquid alloy.
In physics, the maximum bubble pressure method, or in short bubble pressure method, is a technique to measure the surface tension of a liquid, with surfactants.
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.

Wetting solutions are liquids containing active chemical compounds that minimise the distance between two immiscible phases by lowering the surface tension to induce optimal spreading. The two phases, known as an interface, can be classified into five categories, namely, solid-solid, solid-liquid, solid-gas, liquid-liquid and liquid-gas.