
Hydrogen peroxide is a chemical compound with the formula H2O2. In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used both as a monopropellant and an oxidizer in rocketry.

α-Ketoglutaric acid is a keto acid.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.
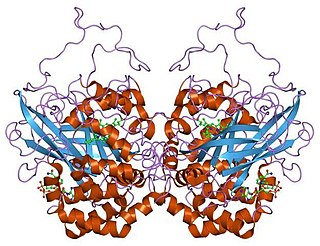
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.

Sorbitol, less commonly known as glucitol, is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol group (−CH2OH). Most sorbitol is made from potato starch, but it is also found in nature, for example in apples, pears, peaches, and prunes. It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2. While similar, the two sugar alcohols have very different sources in nature, melting points, and uses.

Lactic acid is an organic acid. It has the molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate. The name of the derived acyl group is lactoyl.

The sodium–potassium pump is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.

Sodium percarbonate, or sodium carbonate peroxide is a chemical substance with formula Na
2H
3CO
6. It is an adduct of sodium carbonate and hydrogen peroxide whose formula is more properly written as 2 Na
2CO
3 · 3 H
2O
2. It is a colorless, crystalline, hygroscopic and water-soluble solid. It is sometimes abbreviated as SPC. It contains 32.5% by weight of hydrogen peroxide.

Hypochlorous acid is an acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine.

In chemistry and biology, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (O2), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (O2H), superoxide (O2-), hydroxyl radical (OH.), and singlet oxygen. ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.
Tooth whitening or tooth bleaching is the process of lightening the color of human teeth. Whitening is often desirable when teeth become yellowed over time for a number of reasons, and can be achieved by changing the intrinsic or extrinsic color of the tooth enamel. The chemical degradation of the chromogens within or on the tooth is termed as bleaching.
The oxoglutarate dehydrogenase complex (OGDC) or α-ketoglutarate dehydrogenase complex is an enzyme complex, most commonly known for its role in the citric acid cycle.
Dehydroascorbic acid (DHA) is an oxidized form of ascorbic acid. It is actively imported into the endoplasmic reticulum of cells via glucose transporters. It is trapped therein by reduction back to ascorbate by glutathione and other thiols. The (free) chemical radical semidehydroascorbic acid (SDA) also belongs to the group of oxidized ascorbic acids.
In biochemistry, lipogenesis is the conversion of fatty acids and glycerol into fats, or a metabolic process through which acetyl-CoA is converted to triglyceride for storage in fat. Lipogenesis encompasses both fatty acid and triglyceride synthesis, with the latter being the process by which fatty acids are esterified to glycerol before being packaged into very-low-density lipoprotein (VLDL). Fatty acids are produced in the cytoplasm of cells by repeatedly adding two-carbon units to acetyl-CoA. Triacylglycerol synthesis, on the other hand, occurs in the endoplasmic reticulum membrane of cells by bonding three fatty acid molecules to a glycerol molecule. Both processes take place mainly in liver and adipose tissue. Nevertheless, it also occurs to some extent in other tissues such as the gut and kidney. A review on lipogenesis in the brain was published in 2008 by Lopez and Vidal-Puig. After being packaged into VLDL in the liver, the resulting lipoprotein is then secreted directly into the blood for delivery to peripheral tissues.

Crocetin is a natural apocarotenoid dicarboxylic acid that is found in the crocus flower together with its glycoside, crocin, and Gardenia jasminoides fruits. It is also known as crocetic acid. It forms brick red crystals with a melting point of 285 °C.

Uranyl peroxide or uranium peroxide hydrate (UO4·nH2O) is a pale-yellow, soluble peroxide of uranium. It is found to be present at one stage of the enriched uranium fuel cycle and in yellowcake prepared via the in situ leaching and resin ion exchange system. This compound, also expressed as UO3·(H2O2)·(H2O), is very similar to uranium trioxide hydrate UO3·nH2O. The dissolution behaviour of both compounds are very sensitive to the hydration state (n can vary between 0 and 4). One main characteristic of uranium peroxide is that it consists of small needles with an average AMAD of about 1.1 μm.

Sodium ascorbate is one of a number of mineral salts of ascorbic acid (vitamin C). The molecular formula of this chemical compound is C6H7NaO6. As the sodium salt of ascorbic acid, it is known as a mineral ascorbate. It has not been demonstrated to be more bioavailable than any other form of vitamin C supplement.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove colour (whitening) from fabric or fiber or to disinfect after cleaning. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".

Ethyl pyruvate is a colorless organic compound with a molecular formula of C5H8O3.

















