Related Research Articles

Inhalants are a broad range of household and industrial chemicals whose volatile vapors or pressurized gases can be concentrated and breathed in via the nose or mouth to produce intoxication, in a manner not intended by the manufacturer. They are inhaled at room temperature through volatilization or from a pressurized container, and do not include drugs that are sniffed after burning or heating. For example, amyl nitrite (poppers), nitrous oxide and toluene – a solvent widely used in contact cement, permanent markers, and certain types of glue – are considered inhalants, but smoking tobacco, cannabis, and crack cocaine are not, even though these drugs are inhaled as smoke or vapor.

Propane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases. The others include propylene, butane, butylene, butadiene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal.
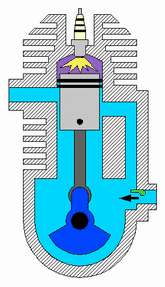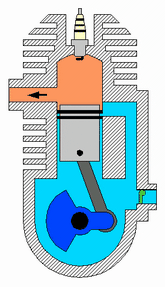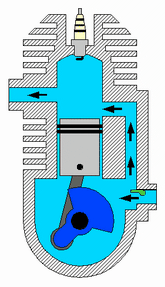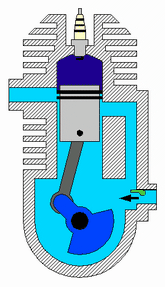
A two-strokeengine is a type of internal combustion engine that completes a power cycle with two strokes of the piston during one power cycle, this power cycle being completed in one revolution of the crankshaft. A four-stroke engine requires four strokes of the piston to complete a power cycle during two crankshaft revolutions. In a two-stroke engine, the end of the combustion stroke and the beginning of the compression stroke happen simultaneously, with the intake and exhaust functions occurring at the same time.
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.
A propellant is a mass that is expelled or expanded in such a way as to create a thrust or another motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicles, the engine that expels the propellant is called a reaction engine. Although technically a propellant is the reaction mass used to create thrust, the term "propellant" is often used to describe a substance which contains both the reaction mass and the fuel that holds the energy used to accelerate the reaction mass. For example, the term "propellant" is often used in chemical rocket design to describe a combined fuel/propellant, although the propellants should not be confused with the fuel that is used by an engine to produce the energy that expels the propellant. Even though the byproducts of substances used as fuel are also often used as a reaction mass to create the thrust, such as with a chemical rocket engine, propellant and fuel are two distinct concepts.

Liquefied petroleum gas is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane, and n-butane.

RP-1 (alternatively, Rocket Propellant-1 or Refined Petroleum-1) is a highly refined form of kerosene outwardly similar to jet fuel, used as rocket fuel. RP-1 provides a lower specific impulse than liquid hydrogen (LH2), but is cheaper, is stable at room temperature, and presents a lower explosion hazard. RP-1 is far denser than LH2, giving it a higher energy density (though its specific energy is lower). RP-1 also has a fraction of the toxicity and carcinogenic hazards of hydrazine, another room-temperature liquid fuel.
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3, (sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications.

Diethyl ether, or simply ether, is an organic compound in the ether class with the formula C4H10O or (C2H5)2O, sometimes abbreviated as Et2O. It is a colourless, highly volatile, sweet-smelling, extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication.

Liquid fuels are combustible or energy-generating molecules that can be harnessed to create mechanical energy, usually producing kinetic energy; they also must take the shape of their container. It is the fumes of liquid fuels that are flammable instead of the fluid. Most liquid fuels in widespread use are derived from fossil fuels; however, there are several types, such as hydrogen fuel, ethanol, and biodiesel, which are also categorized as a liquid fuel. Many liquid fuels play a primary role in transportation and the economy.
Indirect injection in an internal combustion engine is fuel injection where fuel is not directly injected into the combustion chamber.
Homogeneous Charge Compression Ignition (HCCI) is a form of internal combustion in which well-mixed fuel and oxidizer are compressed to the point of auto-ignition. As in other forms of combustion, this exothermic reaction produces heat that can be transformed into work in a heat engine.

The hot-bulb engine is a type of internal combustion engine in which fuel ignites by coming in contact with a red-hot metal surface inside a bulb, followed by the introduction of air (oxygen) compressed into the hot-bulb chamber by the rising piston. There is some ignition when the fuel is introduced, but it quickly uses up the available oxygen in the bulb. Vigorous ignition takes place only when sufficient oxygen is supplied to the hot-bulb chamber on the compression stroke of the engine.

A model engine is a small internal combustion engine typically used to power a radio-controlled aircraft, radio-controlled car, radio-controlled boat, free flight, control line aircraft, or ground-running tether car model.

A carbureted compression ignition model engine, popularly known as a model diesel engine, is a simple compression ignition engine made for model propulsion, usually model aircraft but also model boats. These are quite similar to the typical glow-plug engine that runs on a mixture of methanol-based fuels with a hot wire filament to provide ignition. Despite their name, their use of compression ignition, and the use of a kerosene fuel that is similar to diesel, model diesels share very little with full-size diesel engines.

Butane or n-butane is an alkane with the formula C4H10. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature and pressure. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties, and commercialized by Walter O. Snelling in early 1910s.
Internal combustion engines come in a wide variety of types, but have certain family resemblances, and thus share many common types of components.
Four-stroking is a condition of two-stroke engines where combustion occurs every four strokes or more, rather than every two. Though normal in some instances at idle, extremely high engine speeds, and when letting off the throttle, such firing is uneven, noisy and may, in cases of malfunction, damage the engine if allowed to continue unabated.

An internal combustion engine is a heat engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons, turbine blades, a rotor, or a nozzle. This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to.
References
- ↑ "CRC JUMP START Starting Fluid" (PDF). Archived from the original (PDF) on 2016-08-15. Retrieved 2013-12-17.
- ↑ "NAPA Premium Starting Fluid".
- ↑ "Ethyl Ether MSDS". J.T. Baker. Retrieved 2017-12-28.
- ↑ "The immediate engine damage that can result from ether starting fluid use". The Truck Stop. Retrieved 28 December 2017.
- ↑ "How to Start a Diesel Truck". WikiHow. Retrieved 2 January 2014.
- ↑ Di Justo, Patrick (20 April 2009). "What's Inside WD-40? Superlube's Secret Sauce". Wired . Archived from the original on 19 January 2014. Retrieved 27 May 2020.
- ↑ "Inhalants, inhalant abuse, effects of inhalants at SAMHSA's NCADI". Archived from the original on 2008-12-08. Retrieved 2008-12-08.
- ↑ "medem.com". www.medem.com.