
Streptococcus is a genus of gram-positive coccus or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales, in the phylum Bacillota. Cell division in streptococci occurs along a single axis, so as they grow, they tend to form pairs or chains that may appear bent or twisted. This differs from staphylococci, which divide along multiple axes, thereby generating irregular, grape-like clusters of cells. Most streptococci are oxidase-negative and catalase-negative, and many are facultative anaerobes.

Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause Group A streptococcal infection. S. pyogenes is the predominant species harboring the Lancefield group A antigen, and is often called group A Streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) Streptococcus (GABHS) is thus also used.
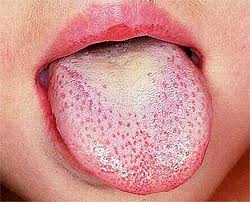
Scarlet fever, also known as scarlatina, is an infectious disease caused by Streptococcus pyogenes, a Group A streptococcus (GAS). The infection is a type of Group A streptococcal infection. It most commonly affects children between five and 15 years of age. The signs and symptoms include a sore throat, fever, headache, swollen lymph nodes, and a characteristic rash. The face is flushed and the rash is red and blanching. It typically feels like sandpaper and the tongue may be red and bumpy. The rash occurs as a result of capillary damage by exotoxins produced by S.pyogenes. On darker-pigmented skin the rash may be hard to discern.

Toxic shock syndrome (TSS) is a condition caused by bacterial toxins. Symptoms may include fever, rash, skin peeling, and low blood pressure. There may also be symptoms related to the specific underlying infection such as mastitis, osteomyelitis, necrotising fasciitis, or pneumonia.
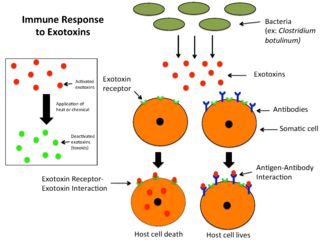
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.

Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically they cause non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. SAgs are produced by some pathogenic viruses and bacteria most likely as a defense mechanism against the immune system. Compared to a normal antigen-induced T-cell response where 0.0001-0.001% of the body's T-cells are activated, these SAgs are capable of activating up to 20% of the body's T-cells. Furthermore, Anti-CD3 and Anti-CD28 antibodies (CD28-SuperMAB) have also shown to be highly potent superantigens.

An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. They can be chromosomally or plasmid encoded. They are heat labile (>60⁰), of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins, secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die.

Lysogeny, or the lysogenic cycle, is one of two cycles of viral reproduction. Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome or formation of a circular replicon in the bacterial cytoplasm. In this condition the bacterium continues to live and reproduce normally, while the bacteriophage lies in a dormant state in the host cell. The genetic material of the bacteriophage, called a prophage, can be transmitted to daughter cells at each subsequent cell division, and later events can release it, causing proliferation of new phages via the lytic cycle. Lysogenic cycles can also occur in eukaryotes, although the method of DNA incorporation is not fully understood. For instance the AIDS viruses can either infect humans lytically, or lay dormant (lysogenic) as part of the infected cells' genome, keeping the ability to return to lysis at a later time. The rest of this article is about lysogeny in bacterial hosts.

Yersinia pseudotuberculosis is a Gram-negative bacterium that causes Far East scarlet-like fever in humans, who occasionally get infected zoonotically, most often through the food-borne route. Animals are also infected by Y. pseudotuberculosis. The bacterium is urease positive.
Virulence factors are cellular structures, molecules and regulatory systems that enable microbial pathogens to achieve the following:
Diphtheria toxin is an exotoxin secreted mainly by Corynebacterium diphtheriae but also by Corynebacterium ulcerans and Corynebacterium pseudotuberculosis. the pathogenic bacterium that causes diphtheria. The toxin gene is encoded by a prophage called corynephage β. The toxin causes the disease in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.

Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternative mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins.
Toxic shock syndrome toxin-1 (TSST-1) is a superantigen with a size of 22 kDa produced by 5 to 25% of Staphylococcus aureus isolates. It causes toxic shock syndrome (TSS) by stimulating the release of large amounts of interleukin-1, interleukin-2 and tumour necrosis factor. In general, the toxin is not produced by bacteria growing in the blood; rather, it is produced at the local site of an infection, and then enters the blood stream.
Streptolysins are two hemolytic exotoxins from Streptococcus. Types include streptolysin O, which is oxygen-labile, and streptolysin S, which is oxygen-stable.

Streptococcus dysgalactiae is a gram positive, beta-haemolytic, coccal bacterium belonging to the family Streptococcaceae. It is capable of infecting both humans and animals, but is most frequently encountered as a commensal of the alimentary tract, genital tract, or less commonly, as a part of the skin flora. The clinical manifestations in human disease range from superficial skin-infections and tonsillitis, to severe necrotising fasciitis and bacteraemia. The incidence of invasive disease has been reported to be rising. Several different animal species are susceptible to infection by S. dysgalactiae, but bovine mastitis and infectious arthritis in lambs have been most frequently reported.
A corynebacteriophage is a DNA-containing bacteriophage specific for bacteria of genus Corynebacterium as its host.

In the field of molecular biology, enterotoxin type B, also known as Staphylococcal enterotoxin B (SEB), is an enterotoxin produced by the gram-positive bacteria Staphylococcus aureus. It is a common cause of food poisoning, with severe diarrhea, nausea and intestinal cramping often starting within a few hours of ingestion. Being quite stable, the toxin may remain active even after the contaminating bacteria are killed. It can withstand boiling at 100 °C for a few minutes. Gastroenteritis occurs because SEB is a superantigen, causing the immune system to release a large amount of cytokines that lead to significant inflammation.
Bacteriophage T12 is a bacteriophage that infects Streptococcus pyogenes bacteria. It is a proposed species of the family Siphoviridae in the order Caudovirales also known as tailed viruses. It converts a harmless strain of bacteria into a virulent strain. It carries the speA gene which codes for erythrogenic toxin A. speA is also known as streptococcal pyogenic exotoxin A, scarlet fever toxin A, or even scarlatinal toxin. Note that the name of the gene "speA" is italicized; the name of the toxin "speA" is not italicized. Erythrogenic toxin A converts a harmless, non-virulent strain of Streptococcus pyogenes to a virulent strain through lysogeny, a life cycle which is characterized by the ability of the genome to become a part of the host cell and be stably maintained there for generations. Phages with a lysogenic life cycle are also called temperate phages. Bacteriophage T12, proposed member of family Siphoviridae including related speA-carrying bacteriophages, is also a prototypic phage for all the speA-carrying phages of Streptococcus pyogenes, meaning that its genome is the prototype for the genomes of all such phages of S. pyogenes. It is the main suspect as the cause of scarlet fever, an infectious disease that affects small children.
RopB transcriptional regulator, also known as RopB/Rgg transcriptional regulator is a transcriptional regulator protein that regulates expression of the extracellularly secreted cysteine protease streptococcal pyrogenic exotoxin B (speB) [See Also: erythrogenic toxins] which is an important virulence factor of Streptococcus pyogenes and is responsible for the dissemination of a host of infectious diseases including strep throat, impetigo, streptococcal toxic shock syndrome, necrotizing fasciitis, and scarlet fever. Functional studies suggest that the ropB multigene regulon is responsible for not only global regulation of virulence but also a wide range of functions from stress response, metabolic function, and two-component signaling. Structural studies implicate ropB's regulatory action being reliant on a complex interaction involving quorum sensing with the leaderless peptide signal speB-inducing peptide (SIP) acting in conjunction with a pH sensitive histidine switch.
Shiranee Sriskandan is a British academic who is Professor of Infectious Diseases at Imperial College London and Honorary Consultant at Hammersmith Hospital. Her research considers how Gram-positive bacteria cause disease, with a particular focus on the bacteria Streptococcus pyogenes.


















