Related Research Articles

α2-Macroglobulin (α2M) or alpha-2-macroglobulin is a large plasma protein found in the blood. It is mainly produced by the liver, and also locally synthesized by macrophages, fibroblasts, and adrenocortical cells. In humans it is encoded by the A2M gene.

Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases. They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site.
High-molecular-weight kininogen is a circulating plasma protein which participates in the initiation of blood coagulation, and in the generation of the vasodilator bradykinin via the kallikrein-kinin system. HMWK is inactive until it either adheres to binding proteins beneath an endothelium disrupted by injury, thereby initiating coagulation; or it binds to intact endothelial cells or platelets for functions other than coagulation.

Cathepsins are proteases found in all animals as well as other organisms. There are approximately a dozen members of this family, which are distinguished by their structure, catalytic mechanism, and which proteins they cleave. Most of the members become activated at the low pH found in lysosomes. Thus, the activity of this family lies almost entirely within those organelles. There are, however, exceptions such as cathepsin K, which works extracellularly after secretion by osteoclasts in bone resorption. Cathepsins have a vital role in mammalian cellular turnover.
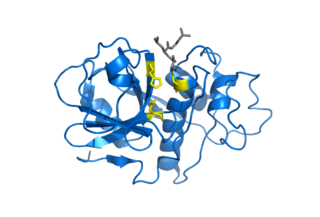
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.

Cathepsin S is a protein that in humans is encoded by the CTSS gene. Transcript variants utilizing alternative polyadenylation signals exist for this gene.
Kininogens are precursor proteins for kinins, biologically active polypeptides involved in blood coagulation, vasodilation, smooth muscle contraction, inflammatory regulation, and the regulation of the cardiovascular and renal systems.

Subtilisin is a protease initially obtained from Bacillus subtilis.

Cathepsin G is a protein that in humans is encoded by the CTSG gene. It is one of the three serine proteases of the chymotrypsin family that are stored in the azurophil granules, and also a member of the peptidase S1 protein family. Cathepsin G plays an important role in eliminating intracellular pathogens and breaking down tissues at inflammatory sites, as well as in anti-inflammatory response.

Cathepsin L1 is a protein that in humans is encoded by the CTSL1 gene. The protein is a cysteine cathepsin, a lysosomal cysteine protease that plays a major role in intracellular protein catabolism.

Cathepsin E is an enzyme that in humans is encoded by the CTSE gene. The enzyme is also known as slow-moving proteinase, erythrocyte membrane aspartic proteinase, SMP, EMAP, non-pepsin proteinase, cathepsin D-like acid proteinase, cathepsin E-like acid proteinase, cathepsin D-type proteinase) is an enzyme.

Streptococcal pyrogenic exotoxins also known as erythrogenic toxins, are exotoxins secreted by strains of the bacterial species Streptococcus pyogenes. SpeA and speC are superantigens, which induce inflammation by nonspecifically activating T cells and stimulating the production of inflammatory cytokines. SpeB, the most abundant streptococcal extracellular protein, is a cysteine protease. Pyrogenic exotoxins are implicated as the causative agent of scarlet fever and streptococcal toxic shock syndrome. There is no consensus on the exact number of pyrogenic exotoxins. Serotypes A, B, and C are the most extensively studied and recognized by all sources, but others note up to thirteen distinct types, categorizing speF through speM as additional superantigens. Erythrogenic toxins are known to damage the plasma membranes of blood capillaries under the skin and produce a red skin rash. Past studies have shown that multiple variants of erythrogenic toxins may be produced, depending on the strain of S. pyogenes in question. Some strains may not produce a detectable toxin at all. Bacteriophage T12 infection of S. pyogenes enables the production of speA, and increases virulence.
IgA protease is an enzyme. This enzyme catalyses the following chemical reaction[reaction equation needed]
Lysyl endopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Endopeptidase La is an enzyme. This enzyme catalyses hydrolysis of proteins in the presence of ATP.

The 3C-like protease (3CLpro) or main protease (Mpro), formally known as C30 endopeptidase or 3-chymotrypsin-like protease, is the main protease found in coronaviruses. It cleaves the coronavirus polyprotein at eleven conserved sites. It is a cysteine protease and a member of the PA clan of proteases. It has a cysteine-histidine catalytic dyad at its active site and cleaves a Gln–(Ser/Ala/Gly) peptide bond.
HIV-2 protease or retropepsin is an enzyme. This enzyme catalyses the following chemical reaction
Vibriolysin is an enzyme. This enzyme catalyses the following chemical reaction
Coccolysin is an enzyme. This enzyme catalyses the following chemical reaction

RopB transcriptional regulator, also known as RopB/Rgg transcriptional regulator is a transcriptional regulator protein that regulates expression of the extracellularly secreted cysteine protease streptococcal pyrogenic exotoxin B, which is an important virulence factor of Streptococcus pyogenes and is responsible for the dissemination of a host of infectious diseases including strep throat, impetigo, streptococcal toxic shock syndrome, necrotizing fasciitis, and scarlet fever. Functional studies suggest that the ropB multigene regulon is responsible for not only global regulation of virulence but also a wide range of functions from stress response, metabolic function, and two-component signaling. Structural studies implicate ropB's regulatory action being reliant on a complex interaction involving quorum sensing with the leaderless peptide signal speB-inducing peptide (SIP) acting in conjunction with a pH sensitive histidine switch.
References
- ↑ Elliott SD, Liu TY (1970). Streptococcal proteinase. Methods Enzymol. Vol. 19. pp. 252–261. doi:10.1016/0076-6879(70)19019-7. ISBN 978-0-12-181881-4.
- ↑ Liu TY, Elliott SD (1971). "Streptococcal proteinase". In Boyer, P.D. (ed.). The Enzymes (3rd ed.). New York: Academic Press. pp. 609–647.
- ↑ Tai JY, Kortt AA, Liu TY, Elliott SD (April 1976). "Primary structure of streptococcal proteinase. III. Isolation of cyanogen bromide peptides: complete covalent structure of the polypeptide chain". The Journal of Biological Chemistry. 251 (7): 1955–9. doi: 10.1016/S0021-9258(17)33640-2 . PMID 1270417.
- ↑ Lo SS, Fraser BA, Liu TY (September 1984). "The mixed disulfide in the zymogen of streptococcal proteinase. Characterization and implication for its biosynthesis". The Journal of Biological Chemistry. 259 (17): 11041–5. doi: 10.1016/S0021-9258(18)90619-8 . PMID 6381494.