Function
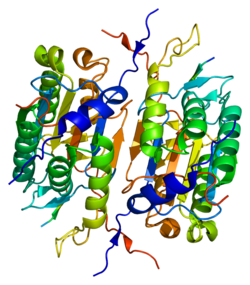
Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes that undergo proteolytic processing at conserved aspartic residues to produce two subunits, large and small, that dimerize to form the active enzyme. The proteolytic cleavage of this protein is induced by a variety of apoptotic stimuli. [7]
Caspase 2 proteolytically cleaves other proteins. It belongs to a family of cysteine proteases called caspases that cleave proteins only at an amino acid following an aspartic acid residue. Within this family, caspase 2 is part of the Ich-1 subfamily. It is one of the most conserved caspases in different species of animal. Caspase 2 has a similar amino acid sequence to initiator caspases, including caspase 1, caspase 4, caspase 5, and caspase 9. It is produced as a zymogen, which contains a long pro-domain that is similar to that of caspase 9 and contains a protein interaction domain known as a CARD domain. Pro-caspase-2 contains two subunits, p19 and p12.
It has been shown to associate with several proteins involved in apoptosis using its CARD domain, including RIP-associated Ich-1/Ced-3-homologue protein with a death domain (RAIDD), apoptosis repressor with caspase recruitment domain (ARC), and death effector filament-forming Ced-4-like apoptosis protein (DEFCAP). [8] Together with RAIDD and p53-induced protein with a death domain ([PIDD])(LRDD), caspase 2 has been shown to form the so-called PIDDosome, [9] which may serve as an activation platform for the protease, although it may also be activated in the absence of PIDD. [10] Overall, caspase 2 appears to be a very versatile caspase with multiple functions beyond cell death induction. [11] [12]
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.