
Keratin is one of a family of fibrous structural proteins. It is the key structural material making up hair, nails, horns, claws, hooves, and the outer layer of human skin. Keratin is also the protein that protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. The only other biological matter known to approximate the toughness of keratinized tissue is chitin.

7-Dehydrocholesterol (7-DHC) is a zoosterol that functions in the serum as a cholesterol precursor, and is converted to vitamin D3 in the skin, therefore functioning as provitamin-D3. The presence of this compound in human skin enables humans to manufacture vitamin D3 (cholecalciferol) from ultraviolet rays in the sun light, via an intermediate isomer pre-vitamin D3. It is also found in the milk of several mammalian species. In insects it is a precursor for the hormone ecdysone, required for reaching adulthood. It was discovered by Nobel-laureate organic chemist Adolf Windaus.

A keratinocyte is the predominant cell type in the epidermis, the outermost layer of the skin, constituting 90% of the cells found there. Those keratinocytes found in the basal layer of the skin are sometimes referred to as "basal cells" or "basal keratinocytes".
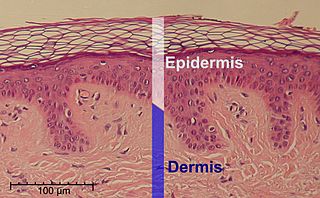
The epidermis is the outermost of the three layers that make up the skin, the inner layers being the dermis and hypodermis. The epidermis layer provides a barrier to infection from environmental pathogens and regulates the amount of water released from the body into the atmosphere through transepidermal water loss. The epidermis is composed of multiple layers of flattened cells that overlie a base layer composed of columnar cells arranged perpendicularly.

The stratum corneum is the outermost layer of the epidermis, consisting of dead cells (corneocytes). This layer is composed of 15–20 layers of flattened cells with no nuclei and cell organelles. Their cytoplasm shows filamentous keratin. These corneocytes are embedded in a lipid matrix composed of ceramides, cholesterol, and fatty acids.
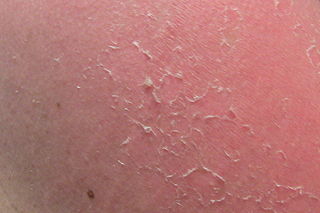
Desquamation, commonly called skin peeling, is the shedding of the outermost membrane or layer of a tissue, such as the skin. The term is from from Latin desquamare, meaning 'to scrape the scales off a fish'.

Hyperkeratosis is thickening of the stratum corneum, often associated with the presence of an abnormal quantity of keratin, and also usually accompanied by an increase in the granular layer. As the corneum layer normally varies greatly in thickness in different sites, some experience is needed to assess minor degrees of hyperkeratosis.

The stratum granulosum is a thin layer of cells in the epidermis. Keratinocytes migrating from the underlying stratum spinosum become known as granular cells in this layer. These cells contain keratohyalin granules, which are filled with histidine- and cysteine-rich proteins that appear to bind the keratin filaments together. Therefore, the main function of keratohyalin granules is to bind intermediate keratin filaments together.

Filaggrin is a filament-associated protein that binds to keratin fibers in epithelial cells. Ten to twelve filaggrin units are post-translationally hydrolyzed from a large profilaggrin precursor protein during terminal differentiation of epidermal cells. In humans, profilaggrin is encoded by the FLG gene, which is part of the S100 fused-type protein (SFTP) family within the epidermal differentiation complex on chromosome 1q21.

The human skin is the outer covering of the body and is the largest organ of the integumentary system. The skin has up to seven layers of ectodermal tissue and guards the underlying muscles, bones, ligaments and internal organs. Human skin is similar to most of the other mammals skin, and human skin is very similar to pig skin. Though nearly all human skin is covered with hair follicles, it can appear hairless. There are two general types of skin, hairy and glabrous skin (hairless). The adjective cutaneous literally means "of the skin".

Protein-glutamine gamma-glutamyltransferase K is an enzyme that in humans is encoded by the TGM1 gene.
Corneocytes are terminally differentiated keratinocytes and compose most if not all of the stratum corneum, the outermost part of the epidermis. They are regularly replaced through desquamation and renewal from lower epidermal layers, making them an essential part of the skin barrier property.

Involucrin is a protein component of human skin and in humans is encoded by the IVL gene. In binding the protein loricrin, involucrin contributes to the formation of a cell envelope that protects corneocytes in the skin.

For the Tetris game, see Tetris: The Grand Master.

Loricrin is a protein that in humans is encoded by the LOR gene.

Corneodesmosin is a protein that in humans is encoded by the CDSN gene.
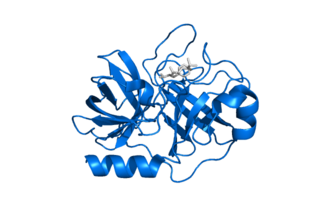
Kallikrein-related peptidase 7 (KLK7) is a serine protease that in humans is encoded by the KLK7 gene. KLK7 was initially purified from the epidermis and characterised as stratum corneum chymotryptic enzyme (SCCE). It was later identified as the seventh member of the human kallikrein family, which includes fifteen homologous serine proteases located on chromosome 19 (19q13).

CYP4F22 is a protein that in humans is encoded by the CYP4F22 gene.

Trichohyalin is a protein that in mammals is encoded by the TCHH gene.













