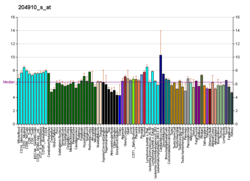
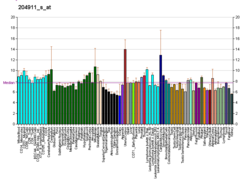
Actinin is a microfilament protein. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. Alpha-actinin is a part of the spectrin superfamily. This superfamily is made of spectrin, dystrophin, and their homologous and isoforms. In non-muscle cells, it is found by the actin filaments and at the adhesion sites.The lattice like arrangement provides stability to the muscle contractile apparatus. Specifically, it helps bind actin filaments to the cell membrane. There is a binding site at each end of the rod and with bundles of actin filaments.

Unconventional myosin-Va is a motor protein in charge of the intracellular transport of vesicles, organelles and protein complexes along the actin filaments. In humans it is coded for by the MYO5A gene.

The interleukin 4 receptor is a type I cytokine receptor. It is a heterodimer, that is, composed of two subunits. IL4R is the human gene coding for IL-4Rα, the subunit which combines with either common gamma chain or with IL-13Rα1.

Interferon-alpha/beta receptor beta chain is a protein that in humans is encoded by the IFNAR2 gene.

Unconventional myosin-VI, is a protein that in humans is coded for by MYO6. Unconventional myosin-VI is a myosin molecular motor involved in intracellular vesicle and organelle transport.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.
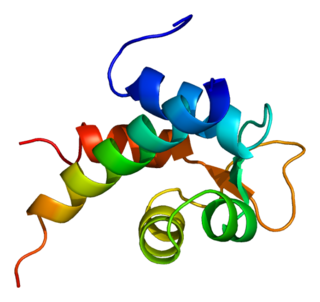
Alpha-actinin-2 is a protein which in humans is encoded by the ACTN2 gene. This gene encodes an alpha-actinin isoform that is expressed in both skeletal and cardiac muscles and functions to anchor myofibrillar actin thin filaments and titin to Z-discs.

Alpha-actinin-4 is a protein that in humans is encoded by the ACTN4 gene.

Tumor necrosis factor, alpha-induced protein 3 or A20 is a protein that in humans is encoded by the TNFAIP3 gene.

Tripartite motif-containing 24 (TRIM24) also known as transcriptional intermediary factor 1α (TIF1α) is a protein that, in humans, is encoded by the TRIM24 gene.

Zinc finger protein RFP is a protein that in humans is encoded by the TRIM27 gene.

Tripartite motif-containing protein 37 is an E3 ubiquitin ligase in humans that is encoded by the TRIM37 gene.

Tripartite motif-containing protein 25 is a protein that in humans is encoded by the TRIM25 gene.

ETS domain-containing protein Elk-3 is a protein that in humans is encoded by the ELK3 gene.

Midline-2 is a protein that in humans is encoded by the MID2 gene.

Tripartite motif-containing protein 32 is a protein that in humans is encoded by the TRIM32 gene. Since its discovery in 1995, TRIM32 has been shown to be implicated in a number of diverse biological pathways.

Tripartite motif-containing protein 68 is a protein that in humans is encoded by the TRIM68 gene.

Tripartite motif-containing protein 9 is a protein that in humans is encoded by the TRIM9 gene.

Tripartite motif-containing protein 55 is a protein that in humans is encoded by the TRIM55 gene.

Tripartite motif-containing protein 15 is a protein that in humans is encoded by the TRIM15 gene.