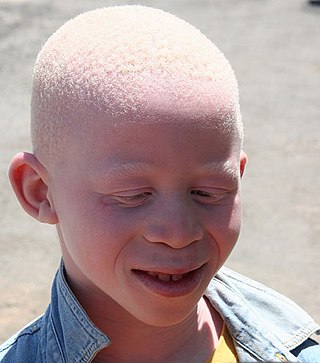
Albinism is a congenital condition characterized in humans by the partial or complete absence of pigment in the skin, hair and eyes. Albinism is associated with a number of vision defects, such as photophobia, nystagmus, and amblyopia. Lack of skin pigmentation makes for more susceptibility to sunburn and skin cancers. In rare cases such as Chédiak–Higashi syndrome, albinism may be associated with deficiencies in the transportation of melanin granules. This also affects essential granules present in immune cells, leading to increased susceptibility to infection.

Human skin color ranges from the darkest brown to the lightest hues. Differences in skin color among individuals is caused by variation in pigmentation, which is the result of genetics, exposure to the sun, disorders, or some combination thereof. Differences across populations evolved through natural selection or sexual selection, because of social norms and differences in environment, as well as regulations of the biochemical effects of ultraviolet radiation penetrating the skin.
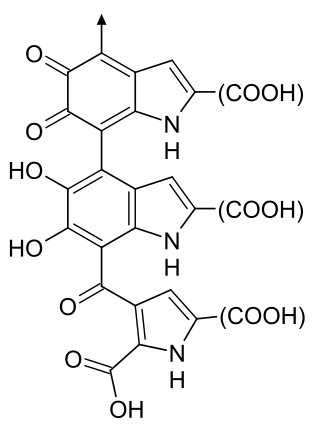
Melanin is a family of biomolecules organized as oligomers or polymers, which among other functions provide the pigments of many organisms. Melanin pigments are produced in a specialized group of cells known as melanocytes.

Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer of the skin's epidermis, the middle layer of the eye, the inner ear, vaginal epithelium, meninges, bones, and heart found in many mammals and birds. Melanin is a dark pigment primarily responsible for skin color. Once synthesized, melanin is contained in special organelles called melanosomes which can be transported to nearby keratinocytes to induce pigmentation. Thus darker skin tones have more melanosomes present than lighter skin tones. Functionally, melanin serves as protection against UV radiation. Melanocytes also have a role in the immune system.

Human hair color is the pigmentation of human hair follicles and shafts due to two types of melanin: eumelanin and pheomelanin. Generally, the more melanin present, the darker the hair. Its tone depends on the ratio of black or brown eumelanin to yellow or red pheomelanin. Melanin levels can vary over time, causing a person's hair color to change, and one person can have hair follicles of more than one color. Some hair colors are associated with some ethnic groups because of the observed higher frequency of particular hair colors within their geographical region, e.g. straight, dark hair amongst East Asians, Southeast Asians, Polynesians, some Central Asians, and Native Americans; a large variety of dark, fair, curly, straight, wavy or bushy amongst Europeans, West Asians, some Central Asians, and North Africans; and curly, dark, and uniquely helical hair amongst Sub Saharan Africans. Bright red hair is found in some European populations, and hair turns gray, white, or "silver" with age.

Equine coat color genetics determine a horse's coat color. Many colors are possible, but all variations are produced by changes in only a few genes. Bay is the most common color of horse, followed by black and chestnut. A change at the agouti locus is capable of turning bay to black, while a mutation at the extension locus can turn bay or black to chestnut.

Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper–Mason pathway. Firstly, the hydroxylation of a monophenol and secondly, the conversion of an o-diphenol to the corresponding o-quinone. o-Quinone undergoes several reactions to eventually form melanin. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin and other pigments from tyrosine by oxidation. It is found inside melanosomes which are synthesized in the skin melanocytes. In humans, the tyrosinase enzyme is encoded by the TYR gene.
Oculocutaneous albinism is a form of albinism involving the eyes, the skin, and the hair. Overall, an estimated 1 in 20,000 people worldwide are born with oculocutaneous albinism. OCA is caused by mutations in several genes that control the synthesis of melanin within the melanocytes. Seven types of oculocutaneous albinism have been described, all caused by a disruption of melanin synthesis and all autosomal recessive disorders. Oculocutaneous albinism is also found in non-human animals.

A white horse is born predominantly white and stays white throughout its life. A white horse has mostly pink skin under its hair coat, and may have brown, blue, or hazel eyes. "True white" horses, especially those that carry one of the dominant white (W) genes, are rare. Most horses that are commonly referred to as "white" are actually "gray" horses whose hair coats are completely white. Gray horses may be born of any color and their hairs gradually turn white as time goes by and take on a white appearance. Nearly all gray horses have dark skin, except under any white markings present at birth. Skin color is the most common method for an observer to distinguish between mature white and gray horses.

Microphthalmia-associated transcription factor also known as class E basic helix-loop-helix protein 32 or bHLHe32 is a protein that in humans is encoded by the MITF gene.

The genetic basis of coat colour in the Labrador Retriever has been found to depend on several distinct genes. The interplay among these genes is used as an example of epistasis.

Dopachrome tautomerase , also known as DCT, is a human gene. Its expression is regulated by the microphthalmia-associated transcription factor (MITF).
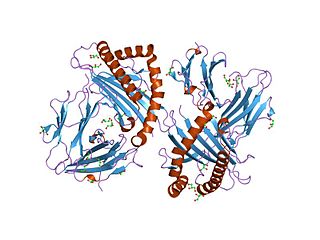
Melanocyte protein PMEL also known as premelanosome protein (PMEL), silver locus protein homolog (SILV) or Glycoprotein 100 (gp100), is a protein that in humans is encoded by the PMEL gene. Its gene product may be referred to as PMEL, silver, ME20, gp100 or Pmel17.

P protein, also known as melanocyte-specific transporter protein or pink-eyed dilution protein homolog, is a protein that in humans is encoded by the oculocutaneous albinism II (OCA2) gene. The P protein is believed to be an integral membrane protein involved in small molecule transport, specifically of tyrosine—a precursor of melanin. Certain mutations in OCA2 result in type 2 oculocutaneous albinism. OCA2 encodes the human homologue of the mouse p gene.

Protein melan-A also known as melanoma antigen recognized by T cells 1 or MART-1 is a protein that in humans is encoded by the MLANA or "MALENA" gene. A fragment of the protein, usually consisting of the nine amino acids 27 to 35, is bound by MHC class I complexes which present it to T cells of the immune system. These complexes can be found on the surface of melanoma cells. Decameric peptides (26-35) are being investigated as cancer vaccines.

Membrane-associated transporter protein (MATP), also known as solute carrier family 45 member 2 (SLC45A2) or melanoma antigen AIM1, is a protein that in humans is encoded by the SLC45A2 gene.
Oculocutaneous albinism type I or type 1A is form of the autosomal recessive condition oculocutaneous albinism that is caused by a dysfunction in the gene for tyrosinase.

Ocular albinism type 1(OA1) is the most common type of ocular albinism, with a prevalence rate of 1:50,000. It is an inheritable classical Mendelian type X-linked recessive disorder wherein the retinal pigment epithelium lacks pigment while hair and skin appear normal. Since it is usually an X-linked disorder, it occurs mostly in males, while females are carriers unless they are homozygous. About 60 missense and nonsense mutations, insertions, and deletions have been identified in Oa1. Mutations in OA1 have been linked to defective glycosylation and thus improper intracellular transportation.

Amelanism is a pigmentation abnormality characterized by the lack of pigments called melanins, commonly associated with a genetic loss of tyrosinase function. Amelanism can affect fish, amphibians, reptiles, birds, and mammals including humans. The appearance of an amelanistic animal depends on the remaining non-melanin pigments. The opposite of amelanism is melanism, a higher percentage of melanin.
The agouti gene, the Agouti-signaling protein (ASIP) is responsible for variations in color in many species. Agouti works with extension to regulate the color of melanin which is produced in hairs. The agouti protein causes red to yellow pheomelanin to be produced, while the competing molecule α-MSH signals production of brown to black eumelanin. In wildtype mice, alternating cycles of agouti and α-MSH production cause agouti coloration. Each hair has bands of yellow which grew during agouti production, and black which grew during α-MSH production. Wildtype mice also have light-colored bellies. The hairs there are a creamy color the whole length because the agouti protein was produced the whole time the hairs were growing.





















