A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Hopanoids are a diverse subclass of triterpenoids with the same hydrocarbon skeleton as the compound hopane. This group of pentacyclic molecules therefore refers to simple hopenes, hopanols and hopanes, but also to extensively functionalized derivatives such as bacteriohopanepolyols (BHPs) and hopanoids covalently attached to lipid A.
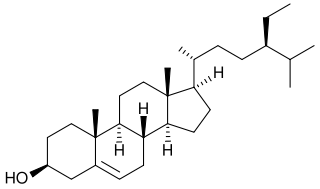
β-sitosterol (beta-sitosterol) is one of several phytosterols with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

Lanosterol synthase is an oxidosqualene cyclase (OSC) enzyme that converts (S)-2,3-oxidosqualene to a protosterol cation and finally to lanosterol. Lanosterol is a key four-ringed intermediate in cholesterol biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.

Prenyltransferases (PTs) are a class of enzymes that transfer allylic prenyl groups to acceptor molecules. Prenyl transferases commonly refer to isoprenyl diphosphate syntheses (IPPSs). Prenyltransferases are a functional category and include several enzyme groups that are evolutionarily independent.
In enzymology, a cycloartenol synthase is an enzyme that catalyzes the chemical reaction
The enzyme amorpha-4,11-diene synthase (ADS) catalyzes the chemical reaction

Ergocryptine is an ergopeptine and one of the ergot alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine.

Capsidiol is a terpenoid compound that accumulates in tobacco Nicotiana tabacum and chili pepper Capsicum annuum in response to fungal infection. Capsidiol is categorized under the broad term of phytoalexin, a class of low molecular weight plant secondary metabolites that are produced during infection. Phytoalexins are also characterized as a part of a two pronged response to infection which involves a short term response consisting of production of free radicals near the site of infection and a long term response involving the production of hormones and an increase in enzymes to biosynthesize phyoalexins such as capsidiol.

Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone, a neurotoxin also found in Artemisia absinthium.

(+)-Costunolide is a naturally occurring sesquiterpene lactone, first isolated in Saussurea costus roots in 1960. It is also found in lettuce.
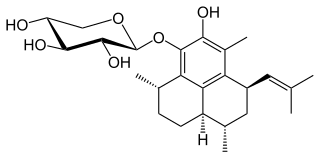
Pseudopterosin A is a diterpene glycoside isolated from the gorgonian sea whip Antillogorgia elisabethae, found in the Bahamas and Florida Keys. Pseudopterosins A-D, which differ in the degree of acetylation at the sugar ring, were first isolated and reported in 1986. There are at least 25 unique diterpenes isolated from this species of marine animal. Samples of P. elisabethae from the Bahamas are found to have higher concentrations of pseudopterosins than populations from the Florida Keys, which have a greater diversity in diterpene structures.
Dammarenediol II synthase (EC 4.2.1.125, dammarenediol synthase, 2,3-oxidosqualene (20S)-dammarenediol cyclase, DDS, (S)-squalene-2,3-epoxide hydro-lyase (dammarenediol-II forming)) is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene hydro-lyase (dammarenediol-II forming). This enzyme catalyses the following chemical reaction
Squalene—hopanol cyclase (EC 4.2.1.129 , squalene—hopene cyclase) is an enzyme with systematic name hopan-22-ol hydro-lyase. This enzyme catalyses the following chemical reaction

Squalene-hopene cyclase (SHC) (EC 5.4.99.17) or hopan-22-ol hydro-lyase is an enzyme in the terpene cyclase/mutase family. It catalyzes the interconversion of squalene into a pentacyclic triterpenes, hopene and hopanol. This enzyme catalyses the following chemical reactions.

Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.