In medicine, a prosthesis, or a prosthetic implant, is an artificial device that replaces a missing body part, which may be lost through physical trauma, disease, or a condition present at birth. Prostheses may restore the normal functions of the missing body part, or may perform a cosmetic function.

Electromyography (EMG) is a technique for evaluating and recording the electrical activity produced by skeletal muscles. EMG is performed using an instrument called an electromyograph to produce a record called an electromyogram. An electromyograph detects the electric potential generated by muscle cells when these cells are electrically or neurologically activated. The signals can be analyzed to detect abnormalities, activation level, or recruitment order, or to analyze the biomechanics of human or animal movement. Needle EMG is an electrodiagnostic medicine technique commonly used by neurologists. Surface EMG is a non-medical procedure used to assess muscle activation by several professionals, including physiotherapists, kinesiologists and biomedical engineers. In computer science, EMG is also used as middleware in gesture recognition towards allowing the input of physical action to a computer as a form of human-computer interaction.

The musculocutaneous nerve is a mixed branch of the lateral cord of the brachial plexus derived from cervical spinal nerves C5-C7. It arises opposite the lower border of the pectoralis minor. It provides motor innervation to the muscles of the anterior compartment of the arm: the coracobrachialis, biceps brachii, and brachialis. It provides sensory innervation to the lateral forearm. It courses through the anterior part of the arm, terminating 2 cm above elbow; after passing the lateral edge of the tendon of biceps brachii it is becomes known as the lateral cutaneous nerve of the forearm.

A nerve conduction study (NCS) is a medical diagnostic test commonly used to evaluate the function, especially the ability of electrical conduction, of the motor and sensory nerves of the human body. These tests may be performed by medical specialists such as clinical neurophysiologists, physical therapists, physiatrists, and neurologists who subspecialize in electrodiagnostic medicine. In the United States, neurologists and physiatrists receive training in electrodiagnostic medicine (performing needle electromyography as part of residency training and, in some cases, acquire additional expertise during a fellowship in clinical neurophysiology, electrodiagnostic medicine, or neuromuscular medicine. Outside the US, clinical neurophysiologists learn needle EMG and NCS testing.
Neuroprosthetics is a discipline related to neuroscience and biomedical engineering concerned with developing neural prostheses. They are sometimes contrasted with a brain–computer interface, which connects the brain to a computer rather than a device meant to replace missing biological functionality.
Jesse Sullivan is an American electrician best known for operating a fully robotic limb through a nerve-muscle graft, making him one of the first non-fictional cyborgs.
Neural engineering is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, or enhance neural systems. Neural engineers are uniquely qualified to solve design problems at the interface of living neural tissue and non-living constructs.

In neuroscience, nerve conduction velocity (CV) is the speed at which an electrochemical impulse propagates down a neural pathway. Conduction velocities are affected by a wide array of factors, which include age, sex, and various medical conditions. Studies allow for better diagnoses of various neuropathies, especially demyelinating diseases as these conditions result in reduced or non-existent conduction velocities. CV is an important aspect of nerve conduction studies.
Extended physiological proprioception (EPP) is a concept pioneered by D.C. Simpson (1972) to describe the ability to perceive at the tip of a tool. Proprioception is the concept is that proprioceptors in the muscles and joints, couple with cutaneous receptors to identify and manage contacts between the body and the world. Extended physiological proprioception allows for this same process to apply to contacts between a tool that is being held and the world. The work was based on prostheses developed at the time in response to disabilities incurred by infants as the result of use of the drug thalidomide by mothers from 1957 to 1962, with the tool in this case simply being the prosthesis itself. How a person identifies with themself changes after a lower limb amputation affects body image, functioning, awareness, and future projections.
Claudia Mitchell is a former United States Marine whose left arm was amputated near the shoulder following a motorcycle crash in 2004. She became the first woman to be outfitted with a bionic arm. The arm is controlled through muscles in her chest and side, which in turn are controlled by the nerves that had previously controlled her real arm. The nerves were rerouted to these muscles in a process of targeted reinnervation.

A traumatic neuroma is a type of neuroma which results from trauma to a nerve, usually during a surgical procedure. The most common oral locations are on the tongue and near the mental foramen of the mouth. They are relatively rare on the head and neck.
Proto 2 is the name of the $55 million initiative of the Defense Advanced Research Projects Agency, or DARPA, to create a thought-controlled prosthetic arm. Its predecessor was called Proto 1 and was capable of reasonably complicated movements like rolling the shoulders, wrists, flexing the fingers. etc.

Shoulder replacement is a surgical procedure in which all or part of the glenohumeral joint is replaced by a prosthetic implant. Such joint replacement surgery generally is conducted to relieve arthritis pain or fix severe physical joint damage.
LifeHand is a prosthetic hand project that allows patients to use an artificial hand to perform daily tasks, but also grants the patient the ability to sense what they are touching. This specific type of prosthetic is called a neuroprosthetic; it's a type of prosthetic that uses a neurological connection between the user and the prosthetic hand to allow the sense of touch. With sensory feedback, the artificial hand allows the user to perceive these senses due to the connection between the patient and the prosthetic. The project was developed/experimented on in the city of Rome, by European researchers from SSSA along with other research centers throughout Europe. There were two LifeHand models that were constructed. The results of the LifeHand experiments were worked on for over a decade and have since been published.
The Michelangelo Hand is a fully articulated robotic hand prosthesis developed by the German prosthetics company Ottobock and its American partner Advanced Arm Dynamics. It is the first prosthesis to feature an electronically actuated thumb which mimics natural human hand movements. The Michelangelo Hand can be used for a variety of delicate everyday tasks, was first fitted to an Austrian elective-amputee in July 2010 and has been in use by military and civilian amputees in the United States and United Kingdom since 2011.
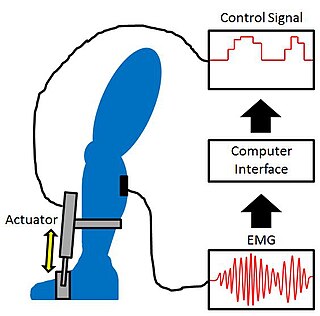
Proportional myoelectric control can be used to activate robotic lower limb exoskeletons. A proportional myoelectric control system utilizes a microcontroller or computer that inputs electromyography (EMG) signals from sensors on the leg muscle(s) and then activates the corresponding joint actuator(s) proportionally to the EMG signal.
A cortical implant is a subset of neuroprosthetics that is in direct connection with the cerebral cortex of the brain. By directly interfacing with different regions of the cortex, the cortical implant can provide stimulation to an immediate area and provide different benefits, depending on its design and placement. A typical cortical implant is an implantable microelectrode array, which is a small device through which a neural signal can be received or transmitted.
Optomyography (OMG) was proposed in 2015 as a technique that could be used to monitor muscular activity. It is possible to use OMG for the same applications where Electromyography (EMG) and Mechanomyography (MMG) are used. However, OMG offers superior signal-to-noise ratio and improved robustness against the disturbing factors and limitations of EMG and MMG. The basic principle of OMG is to use active near-infra-red optical sensors to measure the variations in the measured signals that are reflected from the surface of the skin while activating the muscles below and around the skin spot where the photoelectric sensor is focusing to measure the signals reflected from this spot.
Robotic prosthesis control is a method for controlling a prosthesis in such a way that the controlled robotic prosthesis restores a biologically accurate gait to a person with a loss of limb. This is a special branch of control that has an emphasis on the interaction between humans and robotics.
Álvaro Ríos Poveda is a Colombian electronic engineer, university professor, and researcher who specializes in biomedical engineering and mechatronics. He has performed research on myoelectric prostheses, sensory feedback, and bionic vision technologies.







