
Amputation is the removal of a limb by trauma, medical illness, or surgery. As a surgical measure, it is used to control pain or a disease process in the affected limb, such as malignancy or gangrene. In some cases, it is carried out on individuals as a preventive surgery for such problems. A special case is that of congenital amputation, a congenital disorder, where fetal limbs have been cut off by constrictive bands. In some countries, judicial amputation is currently used to punish people who commit crimes. Amputation has also been used as a tactic in war and acts of terrorism; it may also occur as a war injury. In some cultures and religions, minor amputations or mutilations are considered a ritual accomplishment. When done by a person, the person executing the amputation is an amputator. The oldest evidence of this practice comes from a skeleton found buried in Liang Tebo cave, East Kalimantan, Indonesian Borneo dating back to at least 31,000 years ago, where it was done when the amputee was a young child.

Pain is a distressing feeling often caused by intense or damaging stimuli. The International Association for the Study of Pain defines pain as "an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage."
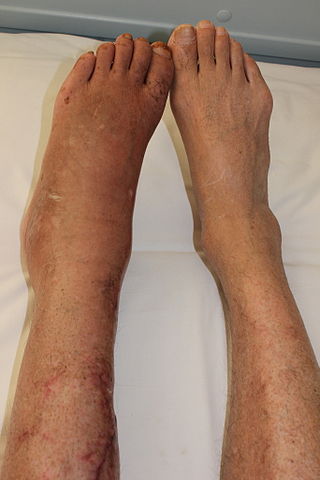
Complex regional pain syndrome, sometimes referred to by the hyponyms reflex sympathetic dystrophy (RSD) or reflex neurovascular dystrophy (RND), is a rare and severe form of neuroinflammatory and dysautonomic disorder causing chronic pain, neurovascular, and neuropathic symptoms. Although it can vary widely, the classic presentation occurs when severe pain from a physical trauma or neurotropic viral infection outlasts the expected recovery time, and may subsequently spread to uninjured areas. The symptoms of types 1 and 2 are the same except type 2 is associated with nerve injury.

Vilayanur Subramanian Ramachandran is an Indian-American neuroscientist. He is known for his wide-ranging experiments and theories in behavioral neurology, including the invention of the mirror box. Ramachandran is a distinguished professor in UCSD's Department of Psychology, where he is the director of the Center for Brain and Cognition.

Sensory neurons, also known as afferent neurons, are neurons in the nervous system, that convert a specific type of stimulus, via their receptors, into action potentials or graded receptor potentials. This process is called sensory transduction. The cell bodies of the sensory neurons are located in the dorsal root ganglia of the spinal cord.
Cortical maps are collections (areas) of minicolumns in the brain cortex that have been identified as performing a specific information processing function.

Mirror therapy (MT) or mirror visual feedback (MVF) is a therapy for pain or disability that affects one side of the patient more than the other side. It was invented by Vilayanur S. Ramachandran to treat post-amputation patients who had phantom limb pain (PLP). Ramachandran created a visual illusion of two intact limbs by putting the patient's affected limb into a "mirror box," with a mirror down the center.
Phantom pain is a painful perception that an individual experiences relating to a limb or an organ that is not physically part of the body, either because it was removed or was never there in the first place.
Neuroplasticity, also known as neural plasticity or just plasticity, is the ability of neural networks in the brain to change through growth and reorganization. Neuroplasticity refers to the brain's ability to reorganize and rewire its neural connections, enabling it to adapt and function in ways that differ from its prior state. This process can occur in response to learning new skills, experiencing environmental changes, recovering from injuries, or adapting to sensory or cognitive deficits. Such adaptability highlights the dynamic and ever-evolving nature of the brain, even into adulthood. These changes range from individual neuron pathways making new connections, to systematic adjustments like cortical remapping or neural oscillation. Other forms of neuroplasticity include homologous area adaptation, cross modal reassignment, map expansion, and compensatory masquerade. Examples of neuroplasticity include circuit and network changes that result from learning a new ability, information acquisition, environmental influences, pregnancy, caloric intake, practice/training, and psychological stress.
Phantom eye syndrome (PES) is a phantom pain in the eye and visual hallucinations after the removal of an eye.
Dysesthesia is an unpleasant, abnormal sense of touch. Its etymology comes from the Greek word "dys," meaning "bad," and "aesthesis," which means "sensation". It often presents as pain but may also present as an inappropriate, but not discomforting, sensation. It is caused by lesions of the nervous system, peripheral or central, and it involves sensations, whether spontaneous or evoked, such as burning, wetness, itching, electric shock, and pins and needles. Dysesthesia can include sensations in any bodily tissue, including most often the mouth, scalp, skin, or legs.
Body schema is an organism's internal model of its own body, including the position of its limbs. The neurologist Sir Henry Head originally defined it as a postural model of the body that actively organizes and modifies 'the impressions produced by incoming sensory impulses in such a way that the final sensation of body position, or of locality, rises into consciousness charged with a relation to something that has happened before'. As a postural model that keeps track of limb position, it plays an important role in control of action.
Tactile discrimination is the ability to differentiate information through the sense of touch. The somatosensory system is the nervous system pathway that is responsible for this essential survival ability used in adaptation. There are various types of tactile discrimination. One of the most well known and most researched is two-point discrimination, the ability to differentiate between two different tactile stimuli which are relatively close together. Other types of discrimination like graphesthesia and spatial discrimination also exist but are not as extensively researched. Tactile discrimination is something that can be stronger or weaker in different people and two major conditions, chronic pain and blindness, can affect it greatly. Blindness increases tactile discrimination abilities which is extremely helpful for tasks like reading braille. In contrast, chronic pain conditions, like arthritis, decrease a person's tactile discrimination. One other major application of tactile discrimination is in new prosthetics and robotics which attempt to mimic the abilities of the human hand. In this case tactile sensors function similarly to mechanoreceptors in a human hand to differentiate tactile stimuli.
Dejerine–Roussy syndrome or thalamic pain syndrome is a condition developed after a thalamic stroke, a stroke causing damage to the thalamus. Ischemic strokes and hemorrhagic strokes can cause lesioning in the thalamus. As initial stroke symptoms dissipate, an imbalance in sensation causes these later syndromes, characterizing Dejerine–Roussy syndrome. Although some treatments exist, they are often expensive, chemically based, invasive, and only treat patients for some time before they need more treatment, called "refractory treatment".

In psychology, visual capture is the dominance of vision over other sense modalities in creating a percept. In this process, the visual senses influence the other parts of the somatosensory system, to result in a perceived environment that is not congruent with the actual stimuli. Through this phenomenon, the visual system is able to disregard what other information a different sensory system is conveying, and provide a logical explanation for whatever output the environment provides. Visual capture allows one to interpret the location of sound as well as the sensation of touch without actually relying on those stimuli but rather creating an output that allows the individual to perceive a coherent environment.
Mirror-touch synesthesia is a rare condition which causes individuals to experience a similar sensation in the same part or opposite part of the body that another person feels. For example, if someone with this condition were to observe someone touching their cheek, they would feel the same sensation on their own cheek. Synesthesia, in general, is described as a condition in which a concept or sensation causes an individual to experience an additional sensation or concept. Synesthesia is usually a developmental condition; however, recent research has shown that mirror touch synesthesia can be acquired after sensory loss following amputation.
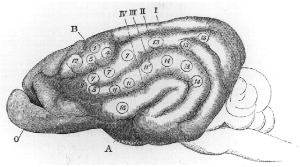
Cortical remapping, also referred to as cortical reorganization, is the process by which an existing cortical map is affected by a stimulus resulting in the creating of a 'new' cortical map. Every part of the body is connected to a corresponding area in the brain which creates a cortical map. When something happens to disrupt the cortical maps such as an amputation or a change in neuronal characteristics, the map is no longer relevant. The part of the brain that is in charge of the amputated limb or neuronal change will be dominated by adjacent cortical regions that are still receiving input, thus creating a remapped area. Remapping can occur in the sensory or motor system. The mechanism for each system may be quite different. Cortical remapping in the somatosensory system happens when there has been a decrease in sensory input to the brain due to deafferentation or amputation, as well as a sensory input increase to an area of the brain. Motor system remapping receives more limited feedback that can be difficult to interpret.

Tactile hallucination is the false perception of tactile sensory input that creates a hallucinatory sensation of physical contact with an imaginary object. It is caused by the faulty integration of the tactile sensory neural signals generated in the spinal cord and the thalamus and sent to the primary somatosensory cortex (SI) and secondary somatosensory cortex (SII). Tactile hallucinations are recurrent symptoms of neurological diseases such as schizophrenia, Parkinson's disease, Ekbom's syndrome and delirium tremens. Patients who experience phantom limb pains also experience a type of tactile hallucination. Tactile hallucinations are also caused by drugs such as cocaine and alcohol.

Limb telescoping is the progressive shortening of a phantom limb as the cortical regions are reorganized following an amputation. During this reorganization, proximal portions of the residual limb are perceived as more distal parts of the phantom limb. Such effect is responsible for increased phantom pain due to the discrepancy between the patient’s body perception and their actual body. This effect may last from weeks up to years after post-amputation.
Dyschiria, also known as dyschiric syndrome, is a neurological disorder where one-half of an individual's body or space cannot be recognized or respond to sensations. The term dyschiria is rarely used in modern scientific research and literature. Dyschiria has been often referred to as unilateral neglect, visuo-spatial neglect, or hemispatial neglect from the 20th century onwards. Psychologists formerly characterized dyschiric patients to be unable to discriminate or report external stimuli. This left the patients incapable of orienting sensory responses in their extrapersonal and personal space. Patients with dyschiria are unable to distinguish one side of their body in general, or specific segments of the body. There are three stages to dyschiria: achiria, allochiria, and synchiria, in which manifestations of dyschiria evolve in varying degrees.
Hanyu-Deutmeyer AA, Cascella M, Varacallo M. Phantom Limb Pain. 2023 Aug 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 28846343.











