In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2023. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.
Diphosphene is a type of organophosphorus compound that has a phosphorus–phosphorus double bond, denoted by R-P=P-R'. These compounds are not common, but their properties have theoretical importance.

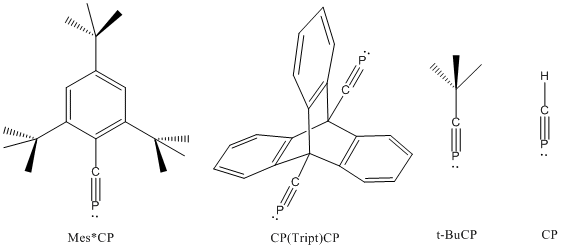
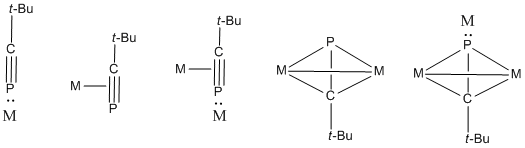
In chemistry, a phosphaalkyne is an organophosphorus compound containing a triple bond between phosphorus and carbon with the general formula R-C≡P. Phosphaalkynes are the heavier congeners of nitriles, though, due to the similar electronegativities of phosphorus and carbon, possess reactivity patterns reminiscent of alkynes. Due to their high reactivity, phosphaalkynes are not found naturally on earth, but the simplest phosphaalkyne, phosphaethyne (H-C≡P) has been observed in the interstellar medium.

In organic chemistry, annulynes or dehydroannulenes are conjugated monocyclic hydrocarbons with alternating single and double bonds in addition to at least one triple bond.
Phosphole is the organic compound with the chemical formula C
4H
4PH; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds.

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.

Group 2 organometallic chemistry refers to the organic derivativess of any group 2 element. It is a subtheme to main group organometallic chemistry. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are typically limited to academic interests.

Disilyne is a low valent silicon compound with the chemical formula Si2R2 where oxidation state of Si is +1. Several isomers are possible, but none are sufficiently stable to be of practical value. Substituted disilynes contain a formal silicon–silicon triple bond and as such are sometimes written R2Si2 (where R is a substituent group). They are the silicon analogues of alkynes.
Methylidynephosphane (phosphaethyne) is a chemical compound which was the first phosphaalkyne compound discovered, containing the unusual C≡P carbon-phosphorus triple bond. Although isolated samples of methylidynephosphane are impractical, other derivatives have been well studied such as tert-butylphosphaacetylene.
Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolated as chemical compounds. Carbenes have some practical utility in organic synthesis but carbene analogs are mostly laboratory curiosities only investigated in academia. Carbene analogs are known for elements of group 13, group 14, group 15 and group 16.

Germylenes are a class of germanium(II) compounds with the general formula :GeR2. They are heavier carbene analogs. However, unlike carbenes, whose ground state can be either singlet or triplet depending on the substituents, germylenes have exclusively a singlet ground state. Unprotected carbene analogs, including germylenes, has a dimerization nature. Free germylenes can be isolated under the stabilization of steric hindrance or electron donation. The synthesis of first stable free dialkyl germylene was reported by Jutzi, et al in 1991.
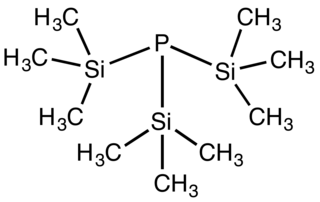
Tris(trimethylsilyl)phosphine is the organophosphorus compound with the formula P(SiMe3)3 (Me = methyl). It is a colorless liquid that ignites in air and hydrolyses readily.

Phosphasilenes or silylidenephosphanes are a class of compounds with silicon-phosphorus double bonds. Since the electronegativity of phosphorus (2.1) is higher than that of silicon (1.9), the "Si=P" moiety of phosphasilene is polarized. The degree of polarization can be tuned by altering the coordination numbers of the Si and P centers, or by modifying the electronic properties of the substituents. The phosphasilene Si=P double bond is highly reactive, yet with the choice of proper substituents, it can be stabilized via donor-acceptor interaction or by steric congestion.

An N-Heterocyclic silylene (NHSi) is a neutral heterocyclic chemical compound consisting of a divalent silicon atom bonded to two nitrogen atoms. The isolation of the first stable NHSi, also the first stable dicoordinate silicon compound, was reported in 1994 by Michael Denk and Robert West three years after Anthony Arduengo first isolated an N-heterocyclic carbene, the lighter congener of NHSis. Since their first isolation, NHSis have been synthesized and studied with both saturated and unsaturated central rings ranging in size from 4 to 6 atoms. The stability of NHSis, especially 6π aromatic unsaturated five-membered examples, make them useful systems to study the structure and reactivity of silylenes and low-valent main group elements in general. Though not used outside of academic settings, complexes containing NHSis are known to be competent catalysts for industrially important reactions. This article focuses on the properties and reactivity of five-membered NHSis.

Arsenic in the solid state can be found as gray, black, or yellow allotropes. These various forms feature diverse structural motifs, with yellow arsenic enabling the widest range of reactivity. In particular, reaction of yellow arsenic with main group and transition metal elements results in compounds with wide-ranging structural motifs, with butterfly, sandwich and realgar-type moieties featuring most prominently.
1-Phosphaallenes is are allenes in which the first carbon atom is replaced by phosphorus, resulting in the structure: -P=C=C<.
Heteroatomic multiple bonding between group 13 and group 15 elements are of great interest in synthetic chemistry due to their isoelectronicity with C-C multiple bonds. Nevertheless, the difference of electronegativity between group 13 and 15 leads to different character of bondings comparing to C-C multiple bonds. Because of the ineffective overlap between p𝝅 orbitals and the inherent lewis acidity/basicity of group 13/15 elements, the synthesis of compounds containing such multiple bonds is challenging and subject to oligomerization. The most common example of compounds with 13/15 group multiple bonds are those with B=N units. The boron-nitrogen-hydride compounds are candidates for hydrogen storage. In contrast, multiple bonding between aluminium and nitrogen Al=N, Gallium and nitrogen (Ga=N), boron and phosphorus (B=P), or boron and arsenic (B=As) are less common.
Pnictogen-substituted tetrahedranes are pnictogen-containing analogues of tetrahedranes with the formula RxCxPn4-x. Computational work has indicated that the incorporation of pnictogens to the tetrahedral core alleviates the ring strain of tetrahedrane. Although theoretical work on pnictogen-substituted tetrahedranes has existed for decades, only the phosphorus-containing species have been synthesized. These species exhibit novel reactivities, most often through ring-opening and polymerization pathways. Phosphatetrahedranes are of interest as new retrons for organophosphorus chemistry. Their strain also make them of interest in the development of energy-dense compounds.