A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Sodium percarbonate or sodium carbonate peroxide is a chemical substance with empirical formula Na2H3CO6. It is an adduct of sodium carbonate and hydrogen peroxide whose formula is more properly written as 2 Na2CO3 · 3 H2O2. It is a colorless, crystalline, hygroscopic and water-soluble solid. It is sometimes abbreviated as SPC. It contains 32.5% by weight of hydrogen peroxide.

Hydrogen peroxide–urea is a white crystalline solid chemical compound composed of equal amounts of hydrogen peroxide and urea. It contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as an oxidizing agent. Often called carbamide peroxide in dentistry, it is used as a source of hydrogen peroxide when dissolved in water for bleaching, disinfection and oxidation.

OxiClean is an American brand of household cleaners, including OxiClean Versatile Stain Remover, which is a laundry additive, spot stain remover, and household cleaner marketed by Church & Dwight. It was formerly owned by Orange Glo International from its introduction in 1997 until it was acquired in 2006.

Laundry detergent is a type of detergent used for cleaning dirty laundry (clothes). Laundry detergent is manufactured in powder and liquid form.
Sodium perborate are chemical compounds with chemical formula [Na+]2[B2O4(OH)4]2−(H2O)x. Commonly encountered salts are the anhydrous form (x = 0) and as a hexahydrate (x = 6). These two species are sometimes called, respectively, "monohydrate" or PBS-1 and "tetrahydrate" or PBS-4, after the historic assumption that NaBO3 would be the anhydrous form). Both the anhydrous and hexahydrate salts are white, odorless, water-soluble solids.
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive.

Sodium persulfate is the inorganic compound with the formula Na2S2O8. It is the sodium salt of peroxydisulfuric acid, H2S2O8, an oxidizing agent. It is a white solid that dissolves in water. It is almost non-hygroscopic and has good shelf-life.
Sodium nonanoyloxybenzenesulfonate (NOBS) is an important component of laundry detergents and bleaches. It is known as a bleach activator for active oxygen sources, allowing formulas containing hydrogen peroxide releasing chemicals to effect bleaching at lower temperatures.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove color from fabric or fiber or to disinfect after cleaning. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
Bleaching of wood pulp is the chemical processing of wood pulp to lighten its color and whiten the pulp. The primary product of wood pulp is paper, for which whiteness is an important characteristic. These processes and chemistry are also applicable to the bleaching of non-wood pulps, such as those made from bamboo or kenaf.
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.

Pyridine-N-oxide is the heterocyclic compound with the formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. It was originally prepared using peroxyacids as the oxidising agent. The compound is used infrequently as an oxidizing reagent in organic synthesis.

Bleach activators are compounds that allow a lower washing temperature than would be required otherwise to achieve the full activity of bleaching agents in the wash liquor. Bleaching agents, usually peroxides, are usually sufficiently active only from 60 °C on. With bleach activators, this activity can already be achieved at lower temperatures. Bleach activators react with hydrogen peroxide in aqueous solution to form peroxy acids. Peroxy acids are more active bleaches than hydrogen peroxide at lower temperatures (<60 °C) but are too unstable to be stored in their active form and hence must be generated in situ.
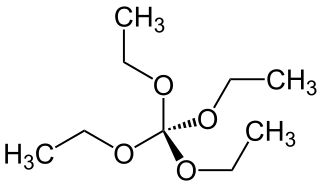
Tetraethoxymethane is a chemical compound which is formally formed by complete ethylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarbonic acid violates the Erlenmeyer rule and is unstable in free state).

In chemistry, metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid. In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metals have a more covalent character.
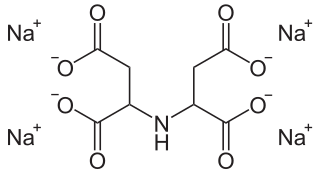
Tetrasodium iminodisuccinate is a sodium salt of iminodisuccinic acid, also referred to as N-(1,2-dicarboxyethyl)aspartic acid.
A peroxide-based bleach or simply peroxide bleach is any bleach product that is based on the peroxide chemical group, namely two oxygen atoms connected by a single bond, (–O–O–). This bond is fairly weak and is often broken in chemical reactions of peroxides, giving rise to very reactive oxygen species, which are the active agents of the bleach.
Peroxynonanoic acid is a peroxycarboxylic acid. It is formed from precursor compounds contained in detergents and acts as a bleach.