
In organic chemistry, a diene ; also diolefin, dy-OH-lə-fin) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alkene units, with the standard prefix di of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition.
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
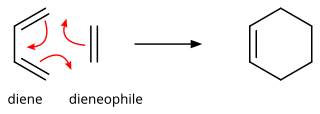
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels–Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.
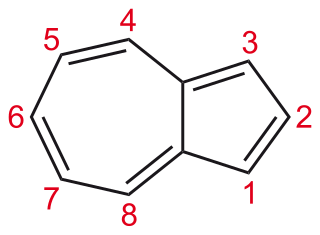
Azulene is an aromatic organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.
In organic chemistry, arynes and benzynes are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of alkynes under high strain.

Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints.
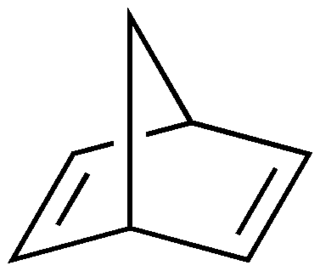
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerize. Norbornadiene is also a useful dienophile in Diels-Alder reactions.
1,3,5-Triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine—the "symmetric" isomer—and its derivatives are useful in a variety of applications.
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium cation is.

Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.

Hexaphenylbenzene is an aromatic compound composed of a benzene ring substituted with six phenyl rings. It is a colorless solid. The compound is the parent member of a wider class of hexaarylbenzenes, which are mainly of theoretical interest.

Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently by Masamune and Dauben and Whalen. A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.
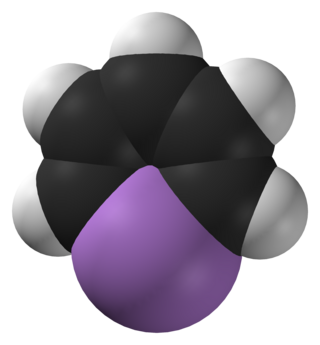
Arsabenzene (IUPAC name: arsinine) is an organoarsenic heterocyclic compound with the chemical formula C5H5As. It belongs to a group of compounds called heteroarenes that have the general formula C5H5E (E= N, P, As, Sb, Bi).

4-Phenyl-1,2,4-triazoline-3,5-dione (PTAD) is an azodicarbonyl compound. PTAD is one of the strongest dienophiles and reacts rapidly with dienes in Diels-Alder reactions. The most prominent use of PTAD was the first synthesis of prismane in 1973.
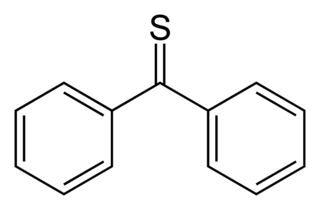
Thiobenzophenone is an organosulfur compound with the formula (C6H5)2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air back to benzophenone and sulfur. Thiobenzophenone is deep blue and dissolves readily in many organic solvents.
The inverse electron demand Diels–Alder reaction, or DAINV or IEDDA is an organic chemical reaction, in which two new chemical bonds and a six-membered ring are formed. It is related to the Diels–Alder reaction, but unlike the Diels–Alder reaction, the DAINV is a cycloaddition between an electron-rich dienophile and an electron-poor diene. During a DAINV reaction, three pi-bonds are broken, and two sigma bonds and one new pi-bond are formed. A prototypical DAINV reaction is shown on the right.
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early and late d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen, nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.
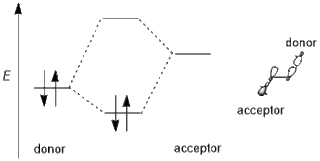
In chemistry, primarily organic and computational chemistry, a stereoelectronic effect is an effect on molecular geometry, reactivity, or physical properties due to spatial relationships in the molecules' electronic structure, in particular the interaction between atomic and/or molecular orbitals. Phrased differently, stereoelectronic effects can also be defined as the geometric constraints placed on the ground and/or transition states of molecules that arise from considerations of orbital overlap. Thus, a stereoelectronic effect explains a particular molecular property or reactivity by invoking stabilizing or destabilizing interactions that depend on the relative orientations of electrons in space.

1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.




















