
Pharmacogenomics is the study of the role of the genome in drug response. Its name reflects its combining of pharmacology and genomics. Pharmacogenomics analyzes how the genetic makeup of a patient affects their response to drugs. It deals with the influence of acquired and inherited genetic variation on drug response, by correlating DNA mutations with pharmacokinetic, pharmacodynamic, and/or immunogenic endpoints.
The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as indexed by Clarivate's Web of Science.

The Zoological Journal of the Linnean Society is a monthly peer-reviewed scientific journal covering zoology published by Oxford University Press on behalf of the Linnean Society. The editor-in-chief is Maarten Christenhusz. It was established in 1856 as the Journal of the Proceedings of the Linnean Society of London. Zoology and renamed Journal of the Linnean Society of London, Zoology in 1866. It obtained its current title in 1969.
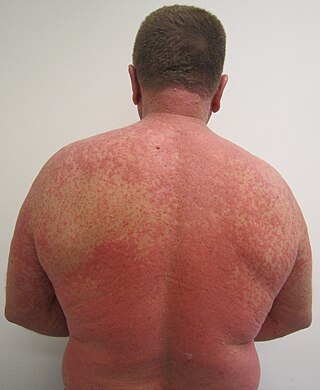
An adverse drug reaction (ADR) is a harmful, unintended result caused by taking medication. ADRs may occur following a single dose or prolonged administration of a drug or result from the combination of two or more drugs. The meaning of this term differs from the term "side effect" because side effects can be beneficial as well as detrimental. The study of ADRs is the concern of the field known as pharmacovigilance. An adverse event (AE) refers to any unexpected and inappropriate occurrence at the time a drug is used, whether or not associated with the administration of the drug. An ADR is a special type of AE in which a causative relationship can be shown. ADRs are only one type of medication-related harm. Another type of medication-related harm type includes not taking prescribed medications, which is also known as non-adherence. Non-adherence to medications can lead to death and other negative outcomes. Adverse drug reactions require the use of a medication.

Google Scholar is a freely accessible web search engine that indexes the full text or metadata of scholarly literature across an array of publishing formats and disciplines. Released in beta in November 2004, the Google Scholar index includes peer-reviewed online academic journals and books, conference papers, theses and dissertations, preprints, abstracts, technical reports, and other scholarly literature, including court opinions and patents.
Genset, a biotechnology company, was established in 1989 in Paris, France with Pascal Brandys as its first president.
Journal Citation Reports (JCR) is an annual publication by Clarivate. It has been integrated with the Web of Science and is accessed from the Web of Science Core Collection. It provides information about academic journals in the natural and social sciences, including impact factors. The JCR was originally published as a part of the Science Citation Index. Currently, the JCR, as a distinct service, is based on citations compiled from the Science Citation Index Expanded and the Social Sciences Citation Index. As of the 2023 edition, journals from the Arts and Humanities Citation Index and the Emerging Sources Citation Index will also be included.

Russ Biagio Altman is an American professor of bioengineering, genetics, medicine, and biomedical data science and past chairman of the bioengineering department at Stanford University.

Pharmaceutical Research is an official journal of the American Association of Pharmaceutical Scientists and covers research spanning the entire spectrum of drug discovery, development, evaluation, and regulatory approval. Small drug molecules, biotechnology products including genes, peptides, proteins and vaccines, and genetically engineered cells are an integral part of papers published. Current emphasis of the journal includes the following areas: preformulation; drug delivery and targeting; formulation design, engineering, and processing; pharmacokinetics, pharmacodynamics, and pharmacogenomics; molecular biopharmaceutics and drug disposition; and computational biopharmaceutics, among others.
Samir Kumar Brahmachari is an Indian biophysicist and Former Director General of the Council of Scientific & Industrial Research (CSIR) and Former Secretary, Department of Scientific and Industrial Research (DSIR), Government of India. He is the Founder Director of Institute of Genomics and Integrative Biology (IGIB), New Delhi and the Chief Mentor of Open Source for Drug Discovery (OSDD) Project. He is the recipient of J.C Bose Fellowship Award, DST (2012). In addition, he is one of the featured researchers in the India Cancer Research Database developed by Institute of Bioinformatics (IOB), Bangalore with support from the Department of Biotechnology, Government of India.
QIAGEN Silicon Valley is a company based in Redwood City, California, USA, that develops software to analyze complex biological systems. QIAGEN Silicon Valley's first product, IPA, was introduced in 2003, and is used to help researchers analyze omics data and model biological systems. The software has been cited in thousands of scientific molecular biology publications and is one of several tools for systems biology researchers and bioinformaticians in drug discovery and institutional research.
Dr. Vinod Scaria FRSB, FRSPH is an Indian biologist, medical researcher pioneering in Precision Medicine and Clinical Genomics in India. He is best known for sequencing the first Indian genome. He was also instrumental in the sequencing of The first Sri Lankan Genome, analysis of the first Malaysian Genome sequencing and analysis of the Wild-type strain of Zebrafish and the IndiGen programme on Genomics for Public Health in India
The Pharmacogenomics Knowledgebase (PharmGKB) is a publicly available, online knowledge base responsible for the aggregation, curation, integration and dissemination of knowledge regarding the impact of human genetic variation on drug response. It is funded by the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS), and is a partner of the NIH Pharmacogenomics Research Network (PGRN). It has been managed at Stanford University since its inception in 2000.
Pharmacogenomics is a peer-reviewed medical journal established in 2000 and published by Future Medicine. The editors-in-chief are David Gurwitz, Howard McLeod, and Munir Pirmohamed. The journal covers the field of pharmacogenomics.
AME Publishing Company is an academic publishing company, which publishes medical journals and books. Founded in July 2009, it is currently headquartered in Hong Kong, with additional offices in Guangzhou, Changsha, Nanjing, Shanghai, Chengdu, Beijing, Taipei, and Hangzhou. Its name stands for "Academic Made Easy/Excellent/Enthusiastic". It has published over 50 medical journals, as well as 20 English-language books, 28 Chinese-language books, and 60 e-books.
Genome Medicine is a peer-reviewed open-access medical journal with a focus on medical genetics. It was established in 2009 as a companion journal to Genome Biology and is published continuously by BioMed Central. The editor-in-chief is Rabia Begum.

Clarivate Plc is a British-American publicly traded analytics company that operates a collection of subscription-based services, in the areas of bibliometrics and scientometrics; business / market intelligence, and competitive profiling for pharmacy and biotech, patents, and regulatory compliance; trademark protection, and domain and brand protection. In the academy and the scientific community, Clarivate is known for being the company which calculates the impact factor, using data from its Web of Science product family, that also includes services/applications such as Publons, EndNote, EndNote Click, and ScholarOne. Its other product families are Cortellis, DRG, CPA Global, Derwent, MarkMonitor, CompuMark, and Darts-ip, and also the various ProQuest products and services.






