
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colourless and odourless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold. It is famous for its extreme oxidation properties. The compound is primarily of interest in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, historically as a component in rocket fuels, and various other industrial operations owing to its corrosive nature.
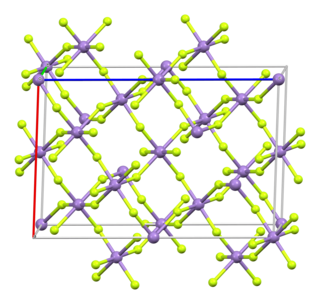
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Nitrogen trifluoride is the inorganic compound with the formula. It is a colorless, non-flammable, toxic gas with a slightly musty odor. In contrast with ammonia, it is nonbasic. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. NF
3 is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of CO
2 when compared over a 100-year period.

Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound - silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Thiazyl fluoride, NSF, is a colourless, pungent gas at room temperature and condenses to a pale yellow liquid at 0.4 °C. Along with thiazyl trifluoride, NSF3, it is an important precursor to sulfur-nitrogen-fluorine compounds. It is notable for its extreme hygroscopicity.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

The tetrafluoroammonium cation is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4, trifluoramine oxide ONF
3, tetrafluoroborate BF−
4 anion and the tetrafluoroberyllate BeF2−
4 anion.

Thionyl tetrafluoride, also known as sulfur tetrafluoride oxide, is an inorganic compound with the formula SOF4. It is a colorless gas.
Iodine monofluoride is an interhalogen compound of iodine and fluorine with formula IF. It is a chocolate-brown solid that decomposes at 0 °C, disproportionating to elemental iodine and iodine pentafluoride:

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
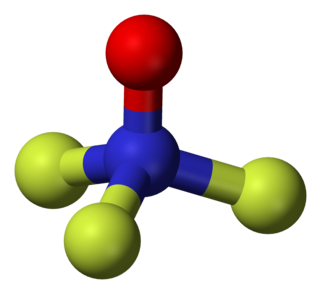
Trifluoramine oxide or Nitrogen trifluoride oxide (F3NO) is an inorganic molecule with strong fluorinating powers.
Thullium(III) fluoride is an inorganic compound with the chemical formula TmF3.

Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3.














