Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.
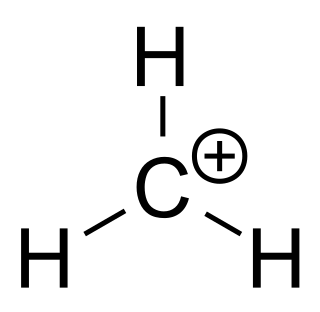
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5 and vinyl C
2H+
3 cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.

The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which a prochiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine reagent which mediates the enantioselective reduction of ketones was previously developed by the laboratory of Itsuno and thus this transformation may more properly be called the Itsuno-Corey oxazaborolidine reduction.

Trimyristin is a saturated fat and the triglyceride of myristic acid with the chemical formula C45H86O6. Trimyristin is a white to yellowish-gray solid that is insoluble in water, but soluble in ethanol, acetone, benzene, chloroform, dichloromethane, ether, and TBME.
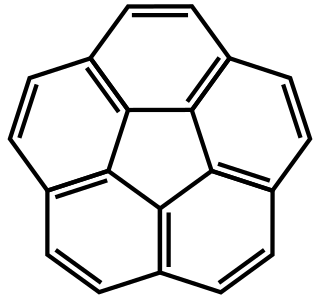
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.

A halonium ion is any onium ion containing a halogen atom carrying a positive charge. This cation has the general structure R−+X−R′ where X is any halogen and no restrictions on R, this structure can be cyclic or an open chain molecular structure. Halonium ions formed from fluorine, chlorine, bromine, and iodine are called fluoronium, chloronium, bromonium, and iodonium, respectively. The 3-membered cyclic variety commonly proposed as intermediates in electrophilic halogenation may be called haliranium ions, using the Hantzsch-Widman nomenclature system.

Methyllithium is the simplest organolithium reagent, with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.
In chemistry and molecular physics, fluxionalmolecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods.

sec-Butyllithium is an organometallic compound with the formula CH3CHLiCH2CH3, abbreviated sec-BuLi or s-BuLi. This chiral organolithium reagent is used as a source of sec-butyl carbanion in organic synthesis.

Metal–organic frameworks (MOFs) are a class of porous polymers consisting of metal clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being 1,4-benzenedicarboxylic acid (BDC).

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are rare and are typically limited to academic interests.

In chemistry, dioxirane is an organic compound with formula CH
2O
2. The molecule consists of a ring with one methylene and two oxygen atoms. It is of interest as the smallest cyclic organic peroxide, but otherwise it is of little practical value.

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
Covalent organic frameworks (COFs) are a class of porous polymers that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic polymers to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties. This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.
Molecular layer deposition (MLD) is a vapour phase thin film deposition technique based on self-limiting surface reactions carried out in a sequential manner. Essentially, MLD resembles the well established technique of atomic layer deposition (ALD) but, whereas ALD is limited to exclusively inorganic coatings, the precursor chemistry in MLD can use small, bifunctional organic molecules as well. This enables, as well as the growth of organic layers in a process similar to polymerization, the linking of both types of building blocks together in a controlled way to build up organic-inorganic hybrid materials.

Mixed valence complexes contain an element which is present in more than one oxidation state. Well-known mixed valence compounds include the Creutz–Taube complex, Prussian blue, and molybdenum blue. Many solids are mixed-valency including indium chalcogenides.















