
Plant viruses are viruses that have the potential to affect plants. Like all other viruses, plant viruses are obligate intracellular parasites that do not have the molecular machinery to replicate without a host. Plant viruses can be pathogenic to vascular plants.

Potyvirus is a genus of positive-strand RNA viruses in the family Potyviridae. Plants serve as natural hosts. Like begomoviruses, members of this genus may cause significant losses in agricultural, pastoral, horticultural, and ornamental crops. More than 200 species of aphids spread potyviruses, and most are from the subfamily Aphidinae. The genus contains 190 species and potyviruses account for about thirty percent of all currently known plant viruses.
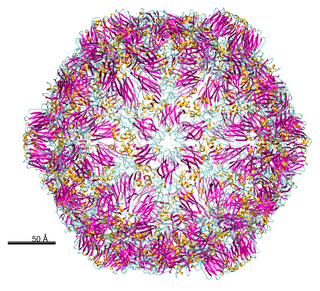
Nepovirus is a genus of viruses in the order Picornavirales, in the family Secoviridae, in the subfamily Comovirinae. Plants serve as natural hosts. There are 40 species in this genus. Nepoviruses, unlike the other two genera in the subfamily Comovirinae, are transmitted by nematodes.
Bean pod mottle virus, or BPMV, is a species of plant pathogenic virus in the family Secoviridae. It is known to infect soybean crops.

Cucumber mosaic virus (CMV) is a plant pathogenic virus in the family Bromoviridae. This virus has a worldwide distribution and a very wide host range, having the reputation of the widest host range of any known plant virus. It can be transmitted from plant to plant both mechanically by sap and by aphids in a stylet-borne fashion. It can also be transmitted in seeds and by the parasitic weeds, Cuscuta sp. (dodder).

Impatiens necrotic spot orthotospovirus(INSV) is a plant pathogenic virus of the order Bunyavirales. It was originally believed to be another strain of Tomato spotted wilt virus, but genetic investigations revealed them to be separate viruses. It is a negative-strand RNA virus which has a tripartite genome. It is largely spread by the insect vector of the western flower thrips. The virus infects more than 648 species of plants including important horticultural and agricultural species such as fuchsia, tomato, orchids, and lettuce (especially romaine). As the name implies, the main symptom on plants is necrotic spots that appear on the leaves. The INSV virus infects by injecting the RNA the virus contains into the cell which then starts using the cell resources to transcribe what the virus RNA states. Viral infection can often result in the death of the plant. The disease is mainly controlled by the elimination of the western flower thrip vector and by destroying any infected plant material.
Potato virus Y (PVY) is a plant pathogenic virus of the family Potyviridae, and one of the most important plant viruses affecting potato production.
Prune dwarf virus (PDV) is an economically important plant pathogenic virus affecting Prunus species globally. PDV is found worldwide due to easy transmission through seed, pollen, and vegetative propagation. The virus is in the family Bromoviridae an important family of plant RNA viruses containing six genera, including Alfamovirus, Ilarvirus, Bromovirus, Amularvirus, Oleavirus, and Cucumovirus. PDV belongs to the genera Ilarvirus. It can cause dwarfism of leaves on certain prune and plum plants. It will also cause yellows in sour cherry, especially when present with Prunus necrotic ringspot virus. There are no known transmission vectors, though the pollen of infected cherry trees has been found to infect other cherry trees a small percent of the time.

Prunus necrotic ringspot virus (PNRSV) is a plant pathogenic virus causing ring spot diseases affecting species of the genus Prunus, as well as other species such as rose and hops. PNRSV is found worldwide due to easy transmission through plant propagation methods and infected seed. The virus is in the family Bromoviridae and genus Ilarvirus. Synonyms of PNRSV include European plum line pattern virus, hop B virus, hop C virus, plum line pattern virus, sour cherry necrotic ringspot virus, and peach ringspot virus.

Tobacco ringspot virus (TRSV) is a plant pathogenic virus in the plant virus family Secoviridae. It is the type species of the genus Nepovirus. Nepoviruses are transmitted between plants by nematodes, thrips, mites, grasshoppers, and flea beetles. TRSV is also easily transmitted by sap inoculation and transmission in seeds has been reported. In recent cases it has also been shown to appear in bees, but no transmission to plants from bees has been noted.
Tomato yellow leaf curl virus (TYLCV) is a DNA virus from the genus Begomovirus and the family Geminiviridae. TYLCV causes the most destructive disease of tomato, and it can be found in tropical and subtropical regions causing severe economic losses. This virus is transmitted by an insect vector from the family Aleyrodidae and order Hemiptera, the whitefly Bemisia tabaci, commonly known as the silverleaf whitefly or the sweet potato whitefly. The primary host for TYLCV is the tomato plant, and other plant hosts where TYLCV infection has been found include eggplants, potatoes, tobacco, beans, and peppers. Due to the rapid spread of TYLCV in the last few decades, there is an increased focus in research trying to understand and control this damaging pathogen. Some interesting findings include the virus being sexually transmitted from infected males to non-infected females, and an evidence that TYLCV is transovarially transmitted to offspring for two generations.

Soybean mosaic virus (SMV) is a member of the plant virus genus Potyvirus. It infects mainly plants belonging to the family Fabaceae but has also been found infecting other economically important crops. SMV is the cause of soybean mosaic disease that occurs in all the soybean production areas of the world. Soybean is one of the most important sources of edible oil and proteins and pathogenic infections are responsible for annual yield losses of about $4 billion in the United States. Among these pathogens, SMV is the most important and prevalent viral pathogen in soybean production worldwide. It causes yield reductions of about 8% to 35%, but losses as high as 94% have been reported.
Strawberry crinkle cytorhabdovirus, commonly called Strawberry crinkle virus (SCV), is a negative sense single stranded RNA virus that threatens strawberry production worldwide. This virus reduces plant rigidity, runner production, fruit size, and production, while causing distortion and crinkling of the leaves. This virus was first described in 1932 in Oregon and California with commercial strawberry varieties, and later became an issue around the world, including North America, South America, Europe, South Africa, New Zealand, Australia, and Japan. Of the family Rhabdoviridae, it is a large family of viruses that affects plants, vertebrates, and invertebrates. Specifically, this virus infects strawberry plants of the genus Fragaria and is transmitted through two aphid vectors that feed on strawberries, Chaetosiphon fragaefolii and C. jacobi. When SCV is combined with other aphid-transmitted strawberry viruses, such as mottle, mild yellow-edge, vein banding, or pallidosis, the damage becomes even more deleterious. Economically, the only significant host of SCV is Fragaria ananassa.

Strawberry vein banding virus (SVBV) is a plant pathogenic virus and a member of the family Caulimoviridae.

Ilarvirus is a genus of positive-strand RNA viruses in the family Bromoviridae. Plants serve as natural hosts. There are 22 species in this genus.

Orthotospovirus is a genus of negative-strand RNA viruses, in the family Tospoviridae of the order Bunyavirales, which infects plants. Tospoviruses take their name from the species Tomato spotted wilt orthotospovirus (TSWV) which was discovered in Australia in 1919. TSWV remained the only known member of the family until the early 1990s when genetic characterisation of plant viruses became more common. There are now at least twenty species in the genus with more being discovered on a regular basis. Member viruses infect over eight hundred plant species from 82 different families.
Soybean vein necrosis orthotospovirus is a plant pathogenic virus of soybeans. SVNV is a relatively new virus, which was discovered in Tennessee in 2008 and has recently been found in many US states from the Southeast and East coast to some western states including CA. This pathogen initially causes intraveinal chlorosis (yellowing) in leaves. This chlorosis then spreads throughout the leaf and eventually these chlorotic areas can become necrotic. It is a member of the order Bunyavirales, family Tospoviridae and genus Orthotospovirus, which is the only genus within this virus family that infects plants. Like other members of Bunyavirales, this virus is enveloped and has a negative sense single-stranded RNA (−ssRNA) genome composed of three genomic segments. It encodes proteins on the M and S segments in an ambisense manner.
Cassava brown streak virus is a species of positive-strand RNA viruses in the genus Ipomovirus and family Potyviridae which infects plants. Member viruses are unique in their induction of pinwheel, or scroll-shaped inclusion bodies in the cytoplasm of infected cells. Cylindrical inclusion bodies include aggregations of virus-encoded helicase proteins. These inclusion bodies are thought to be sites of viral replication and assembly, making then an important factor in the viral lifecycle. Viruses from both the species Cassava brown streak virus and Ugandan cassava brown streak virus (UCBSV), lead to the development of Cassava Brown Streak Disease (CBSD) within cassava plants.

Carnation Italian Ringspot Virus (CIRV) is a plant virus that impacts carnation plants. These flowers are a popular choice in ornamental flower arrangements. This article will provide an overview of CIRV. This will include the history of the virus, information on transmission, symptoms, and characteristics, and research about how it relates to plant physiology.
Carrot virus Y (CarVY) is a (+)ss-RNA virus that affects crops of the carrot family (Apiaceae), such as carrots, anise, chervil, coriander, cumin, dill and parsnip. Carrots are the only known crop to be infected in the field. Infection by the virus leads to deformed roots and discolored or mottled leaves. The virus is spread through insect vectors, and is currently only found in Australia.












