
Benzoic acid is a white solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring with a carboxyl substituent. The benzoyl group is often abbreviated "Bz", thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.

Curcumin is a bright yellow chemical produced by plants of the Curcuma longa species. It is the principal curcuminoid of turmeric, a member of the ginger family, Zingiberaceae. It is sold as a herbal supplement, cosmetics ingredient, food flavoring, and food coloring.

Cinnamaldehyde is an organic compound with the formula() C6H5CH=CHCHO. Occurring naturally as predominantly the trans (E) isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene.

Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile.

Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid or α-substituted β-aryl acrylic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)COOH. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

Dibenzylideneacetone or dibenzalacetone, often abbreviated dba, is an organic compound with the formula C17H14O. It is a pale-yellow solid insoluble in water, but soluble in ethanol.
Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ultraviolet lamps are employed. Organic photochemistry has proven to be a very useful synthetic tool. Complex organic products can be obtained simply.
The Barton reaction, also known as the Barton nitrite ester reaction, is a photochemical reaction that involves the photolysis of an alkyl nitrite to form a δ-nitroso alcohol.
The Varrentrapp reaction, also named Varrentrapp degradation, is a name reaction in the organic chemistry. It is named after Franz Varrentrapp, who described this reaction in 1840. The reaction entails the degradation of an unsaturated carboxylic acid into a saturated acid with two fewer carbon atoms and acetic acid. The fragmentation is induced by action of molten alkali.
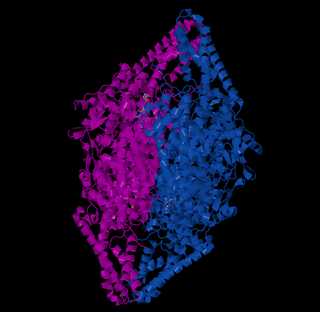
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and trans-cinnamic acid.:
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarine.

Truxillic acids are any of several crystalline stereoisomeric cyclic dicarboxylic acids with the formula (C6H5C2H2(CO2H)2. They are colorless solids. These compounds are obtained by the [2 + 2] photocycloadditions of cinnamic acid where the two trans alkenes react head-to-tail. The isolated stereoisomers are called truxillic acids. The preparation of truxillic acids provided an early example of organic photochemistry.

Photogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition.














