Materials
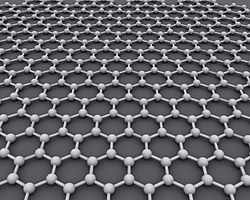
Graphene
Graphene, consisting of single sheets of carbon atoms, has high electron mobility and high thermal conductivity. One issue regarding graphene is its lack of a band gap, which poses a problem in particular with digital electronics because it is unable to switch off field-effect transistors (FETs). [3]

Hexagonal boron nitride
Monolayer hexagonal boron nitride (h-BN) is an insulator with a high energy gap (5.97 eV). [5] However, it can also function as a semiconductor with enhanced conductivity due to its zigzag sharp edges and vacancies. h-BN is often used as substrate and barrier due to its insulating property. h-BN also has a large thermal conductivity.
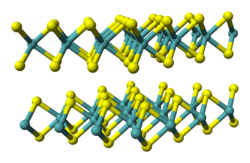
Transition-metal dichalcogenides
Stacks of two-dimensional materials held together by van der Waals forces can form van der Waals heterostructures, in which each atomic sheet simultaneously serves as the bulk and the interface. Interlayer charge transfer, proximity coupling, and moiré superlattice reconstruction lead to emergent properties such as secondary Dirac points in graphene/hBN and enhanced spin–orbit interaction in graphene adjacent to Transition-metal dichalcogenides. [6]
Transition-metal dichalcogenide monolayers (TMDs or TMDCs) are a class of two-dimensional materials that have the chemical formula MX2, where M represents transition metals from group IV, V and VI, and X represents a chalcogen such as sulfur, selenium or tellurium. [7] MoS2, MoSe2, MoTe2, WS2 and WSe2 are TMDCs. TMDCs have layered structure with a plane of metal atoms in between two planes of chalcogen atoms as shown in Figure 1. Each layer is bonded strongly in plane, but weakly in interlayers. Therefore, TMDCs can be easily exfoliated into atomically thin layers through various methods. TMDCs show layer-dependent optical and electrical properties. When exfoliated into monolayers, the band gaps of several TMDCs change from indirect to direct, [8] which lead to broad applications in nanoelectronics, [3] optoelectronics, [9] [10] and quantum computing. [11] While exfoliated TMDC monolayers exhibit promising optoelectronic properties, they are often limited by intrinsic and extrinsic defects, [12] such as sulfur vacancies and grain boundaries, which can negatively affect their performance. To address these issues, various chemical passivation techniques, including the use of superacids and thiol molecules, [13] have been developed to enhance their photoluminescence and charge transport properties. Additionally, phase [14] and strain engineering [15] have emerged as powerful strategies to further optimize the electronic characteristics of TMDCs, making them more suitable for advanced applications in nanoelectronics and quantum computing.
III-VI chalcogenides
Another class of 2D semiconductors are III-VI chalcogenides. These materials have the chemical formula MX, where M is a metal from group 13 (Ga, In) and X is a chalcogen atom (S, Se, Te). Typical members of this group are InSe and GaSe, both of which have shown high electronic mobilities and band gaps suitable for a wide range of electronic applications. [16] [17]


