
Ubiquitin is a small regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ubiquitously. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A.

The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules.
An epitope, also known as antigenic determinant, is the part of an antigen that is recognized by the immune system, specifically by antibodies, B cells, or T cells. The epitope is the specific piece of the antigen to which an antibody binds. The part of an antibody that binds to the epitope is called a paratope. Although epitopes are usually non-self proteins, sequences derived from the host that can be recognized are also epitopes.
Angiostatin is a naturally occurring protein found in several animal species, including humans. It is an endogenous angiogenesis inhibitor. Clinical trials have been undertaken for its use in anticancer therapy.

Plasmin is an important enzyme present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.

Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically it causes non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. SAgs are produced by some pathogenic viruses and bacteria most likely as a defense mechanism against the immune system. Compared to a normal antigen-induced T-cell response where 0.0001-0.001% of the body's T-cells are activated, these SAgs are capable of activating up to 20% of the body's T-cells. Furthermore, Anti-CD3 and Anti-CD28 antibodies (CD28-SuperMAB) have also shown to be highly potent superantigens.

A peptidomimetic is a small protein-like chain designed to mimic a peptide. They typically arise either from modification of an existing peptide, or by designing similar systems that mimic peptides, such as peptoids and β-peptides. Irrespective of the approach, the altered chemical structure is designed to advantageously adjust the molecular properties such as stability or biological activity. This can have a role in the development of drug-like compounds from existing peptides. These modifications involve changes to the peptide that will not occur naturally. Based on their similarity with the precursor peptide, peptidomimetics can be grouped into four classes where A features the most and D the least similarities. Classes A and B involve peptide-like scaffolds, while classes C and D include small molecules.
Antigen processing, or the cytosolic pathway, is an immunological process that prepares antigens for presentation to special cells of the immune system called T lymphocytes. It is considered to be a stage of antigen presentation pathways. This process involves two distinct pathways for processing of antigens from an organism's own (self) proteins or intracellular pathogens, or from phagocytosed pathogens ; subsequent presentation of these antigens on class I or class II major histocompatibility complex (MHC) molecules is dependent on which pathway is used. Both MHC class I and II are required to bind antigen before they are stably expressed on a cell surface. MHC I antigen presentation typically involves the endogenous pathway of antigen processing, and MHC II antigen presentation involves the exogenous pathway of antigen processing. Cross-presentation involves parts of the exogenous and the endogenous pathways but ultimately involves the latter portion of the endogenous pathway.

Angiomotin (AMOT) is a protein that in humans is encoded by the AMOT gene. It belongs to the motin family of angiostatin binding proteins, which includes angiomotin, angiomotin-like 1 (AMOTL1) and angiomotin-like 2 (AMOTL2) characterized by coiled-coil domains at N-terminus and consensus PDZ-binding domain at the C-terminus. Angiomotin is expressed predominantly in endothelial cells of capillaries as well as angiogenic tissues such as placenta and solid tumor.
Dendrotoxins are a class of presynaptic neurotoxins produced by mamba snakes (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel proteins.

Kringle Domains are autonomous protein domains that fold into large loops stabilized by 3 disulfide linkages. These are important in protein–protein interactions with blood coagulation factors. The name Kringle comes from the Scandinavian pastry that these structures resemble.

ICAM-1 also known as CD54 is a protein that in humans is encoded by the ICAM1 gene. This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. It binds to integrins of type CD11a / CD18, or CD11b / CD18 and is also exploited by rhinovirus as a receptor for entry into respiratory epithelium.

Diphtheria toxin is an exotoxin secreted by Corynebacterium, the pathogenic bacterium that causes diphtheria. The toxin gene is encoded by a prophage called corynephage β. The toxin causes the disease in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.
Molecular mimicry is defined as the theoretical possibility that sequence similarities between foreign and self-peptides are sufficient to result in the cross-activation of autoreactive T or B cells by pathogen-derived peptides. Despite the prevalence of several peptide sequences which can be both foreign and self in nature, a single antibody or TCR can be activated by just a few crucial residues which stresses the importance of structural homology in the theory of molecular mimicry. Upon the activation of B or T cells, it is believed that these "peptide mimic" specific T or B cells can cross-react with self-epitopes, thus leading to tissue pathology (autoimmunity). Molecular mimicry is a phenomenon that has been just recently discovered as one of several ways in which autoimmunity can be evoked. A molecular mimicking event is, however, more than an epiphenomenon despite its low statistical probability of occurring and these events have serious implications in the onset of many human autoimmune disorders.

Thrombospondin 1, abbreviated as THBS1, is a protein that in humans is encoded by the THBS1 gene.
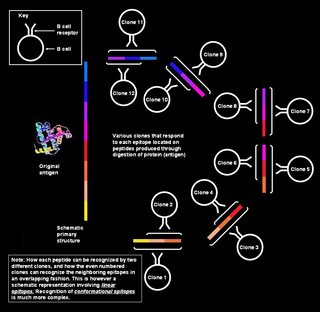
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts, called epitopes, by multiple clones of B cell.

Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternative mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins.
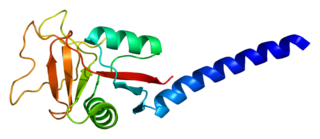
Tetranectin is a protein that in humans is encoded by the CLEC3B gene.
Avimers are artificial proteins that are able to specifically bind to certain antigens via multiple binding sites. Since they are not structurally related to antibodies, they are classified as a type of antibody mimetic. Avimers have been developed by the biotechnology company Avidia, now part of Amgen, as potential new pharmaceutical drugs.
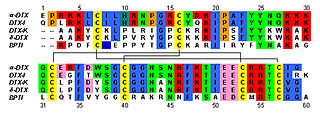
Conantokins are a small family of helical peptides that are derived from the venom of predatory marine snails of the genus Conus. Conantokins act as potent and specific antagonists of the N-methyl-D-aspartate receptor (NMDAR). They are the only naturally-derived peptides to do so. The subtypes of conantokins exhibit a surprising variability of selectivity across the NMDAR subunits, and are therefore uniquely useful in developing subunit-specific pharmacological probes.














