Related Research Articles

The 1957–1958 Asian flu pandemic was a global pandemic of influenza A virus subtype H2N2 that originated in Guizhou in Southern China. The number of excess deaths caused by the pandemic is estimated to be 1–4 million around the world, making it one of the deadliest pandemics in history. A decade later, a reassorted viral strain H3N2 further caused the Hong Kong flu pandemic (1968–1969).

Vaccine diplomacy, a form of medical diplomacy, is the use of vaccines to improve a country's diplomatic relationship and influence of other countries. Meanwhile, vaccine diplomacy also "means a set of diplomatic measures taken to ensure access to the best practices in the development of potential vaccines, to enhance bilateral and/or multilateral cooperation between countries in conducting joint R&D, and, in the case of the announcement of production, to ensure the signing of a contract for the purchase of the vaccine at the shortest term." Although primary discussed in the context of the supply of COVID-19 vaccines, it also played a part in the distribution of the smallpox vaccine.

CoronaVac, also known as the Sinovac COVID-19 vaccine, is a whole inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. It was phase III clinically trialled in Brazil, Chile, Indonesia, the Philippines, and Turkey and relies on traditional technology similar to other inactivated-virus COVID-19 vaccines, such as the Sinopharm BIBP vaccine, another Chinese vaccine, and Covaxin, an Indian vaccine. CoronaVac does not need to be frozen, and both the final product and the raw material for formulating CoronaVac can be transported refrigerated at 2–8 °C (36–46 °F), the temperatures at which flu vaccines are kept.
The COVID-19 vaccination program in the Philippines is an ongoing mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. The vaccination program was initiated by the Duterte administration on March 1, 2021, a day after the arrival of the country's first vaccine doses which were donated by the Chinese government.
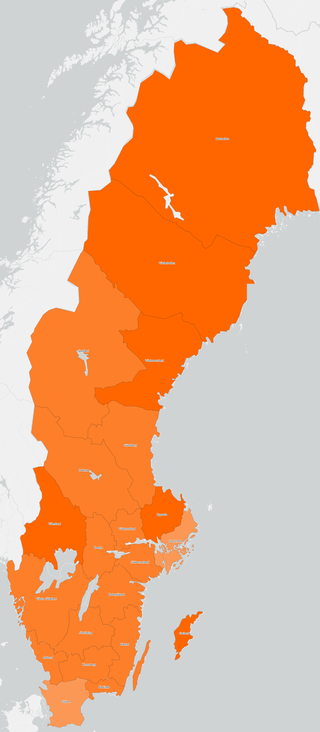
Vaccination against COVID-19 in Sweden started on 27 December 2020 after the approval of the Pfizer–BioNTech vaccine by the European Commission. In Sweden, the Public Health Agency has been commissioned by the government to create a vaccination plan. Sveriges riksbank, the central bank of Sweden, predicts that efficient vaccination against COVID-19 has macroeconomic benefits. As of 20 April 2022, 87.1% of people in Sweden have received at least one dose, with a total of 21,491,717 doses administered. At least one vaccine has been approved for all age groups 12 and older. Children younger than 12 in high risk groups can also be vaccinated.

Israel's COVID-19 vaccination programme, officially named "Give a Shoulder", began on 19 December 2020, and has been praised for its speed, having given twenty percent of the Israeli population the first dose of the vaccines' two dose regimen in the span of three weeks.

India began administration of COVID-19 vaccines on 16 January 2021. As of 4 March 2023, India has administered over 2.2 billion doses overall, including first, second and precautionary (booster) doses of the currently approved vaccines. In India, 95% of the eligible population (12+) has received at least one shot, and 88% of the eligible population (12+) is fully vaccinated.

As of 3 January 2024, 13.53 billion COVID-19 vaccine doses have been administered worldwide, with 70.6 percent of the global population having received at least one dose. While 4.19 million vaccines were then being administered daily, only 22.3 percent of people in low-income countries had received at least a first vaccine by September 2022, according to official reports from national health agencies, which are collated by Our World in Data.

The general COVID-19 vaccination in Australia program began on 22 February 2021 in response to the COVID-19 pandemic, with the goal of vaccinating all willing people in Australia before 2022. Front-line workers and aged care staff and residents had priority for being inoculated, before a gradual phased release to less-vulnerable and lower-risk population groups throughout 2021. The Therapeutic Goods Administration (TGA) approved four vaccines for Australian use in 2021: the Pfizer–BioNTech vaccine on 25 January, the Oxford–AstraZeneca vaccine on 16 February, Janssen vaccine on 25 June and the Moderna vaccine on 9 August. Although approved for use, the Janssen vaccine was not included in the Australian vaccination program as of June 2021.

COVID-19 vaccination in Canada is an ongoing, intergovernmental effort coordinated between the bodies responsible in the Government of Canada to acquire and distribute vaccines to individual provincial and territorial governments who in turn administer authorized COVID-19 vaccines during the COVID-19 pandemic in Canada. Provinces have worked with local municipal governments, hospital systems, family doctors and independently owned pharmacies to aid in part, or in full with vaccination rollout. The vaccination effort in full is the largest such immunization effort in the nation's history. The vaccination effort began December 14, 2020, and is currently ongoing.

The National COVID-19 Immunisation Programme, abbreviated as NIP or PICK, is a national vaccination campaign that is currently being implemented by the Malaysian government as an approach in curbing the spread of coronavirus disease 2019 (COVID-19) and to end the COVID-19 pandemic in Malaysia by successfully achieving the highest immunisation rate among its citizens and non-citizens that are residing in Malaysia. It is the largest immunisation programme implemented in the history of the country, and it is being administered by the Special Committee for Ensuring Access to COVID-19 Vaccine Supply (JKJAV) since early 2021.
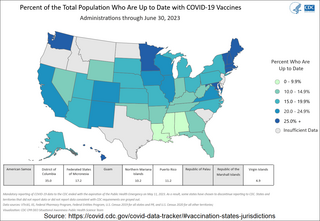
The COVID-19 vaccination campaign in the United States is an ongoing mass immunization campaign for the COVID-19 pandemic in the United States. The Food and Drug Administration (FDA) first granted emergency use authorization to the Pfizer–BioNTech vaccine on December 10, 2020, and mass vaccinations began four days later. The Moderna vaccine was granted emergency use authorization on December 17, 2020, and the Janssen vaccine was granted emergency use authorization on February 27, 2021. By April 19, 2021, all U.S. states had opened vaccine eligibility to residents aged 16 and over. On May 10, 2021, the FDA approved the Pfizer-BioNTech vaccine for adolescents aged 12 to 15. On August 23, 2021, the FDA granted full approval to the Pfizer–BioNTech vaccine for individuals aged 16 and over.
The COVID-19 vaccination campaign in Quebec is an ongoing provincial effort to distribute and administer vaccines against COVID-19.

The COVID-19 vaccination campaign in Germany began on 26 December 2020.
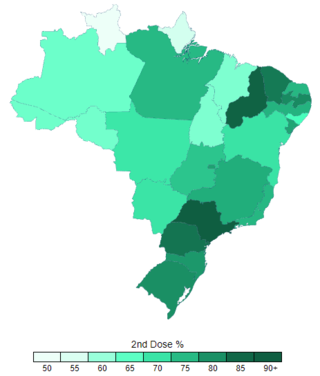
The COVID-19 vaccination campaign in Brazil is an ongoing mass immunization campaign for the COVID-19 pandemic in Brazil. It started on January 17, 2021, when the country had 210 thousand deaths.

Nepal began administration of COVID-19 vaccines on 27 January 2021. 1 million Oxford-Astrazeneca vaccines were provided by India as a grant while Nepal brought 2 million doses from Serum Institute of India (SII) and was one of the first to receive COVID-19 vaccines. The delivery of the first 1 million doses arrived on 21 February. In March, India's decision to ban exports of vaccines created uncertainty over whether Nepal would be able to continue its vaccinations. By April, SII had only provided half of the 2 million doses for which Nepal had paid in full. A spokesperson for the Indian Ministry of External Affairs rejected the notion of an export ban and said "We will export vaccines taking into account the domestic demand." By late July, there was still uncertainty in Nepal over when SII would deliver the vaccines that were purchased, although Prime Minister Narendra Modi said India would "resume the supply of vaccines soon."

COVID-19 vaccination in Sri Lanka is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, in response to the ongoing pandemic in the country. As of late July, the Sinopharm BIBP vaccine accounted for 78% of the total 13.8 million vaccines obtained by Sri Lanka to date. The United States donated over 1.5 million Moderna vaccine through COVAX.

The COVID-19 vaccination in Spain is the national vaccination strategy started on 27 December 2020 in order to vaccinate the country's population against COVID-19 within the international effort to fight the COVID-19 pandemic.

Anti-vaccination activists and other people in many countries have spread a variety of unfounded conspiracy theories and other misinformation about COVID-19 vaccines based on misunderstood or misrepresented science, religion, and law. These have included exaggerated claims about side effects, misrepresentations about how the immune system works and when and how COVID-19 vaccines are made, a story about COVID-19 being spread by 5G, and other false or distorted information. This misinformation has proliferated and may have made many people averse to vaccination. This has led to governments and private organizations around the world introducing measures to incentivize or coerce vaccination, such as lotteries, mandates, and free entry to events, which has in turn led to further misinformation about the legality and effect of these measures themselves.
COVID-19 vaccination in Ontario began in December 2020, when the first doses of the Pfizer vaccine were administered. In February 2021, shipments for both the Pfizer and Moderna vaccines increased significantly. By May 2021, over 50 percent of Ontarians had received their first dose. By the beginning of 2022, over 80 percent of Ontarians had received their first dose.
References
- ↑ Young, Katharine (11 March 2021). "Skipping the Vaccine Line Is Not Only Unethical –It May Undermine Trust in the Rollout". The Conversation. Archived from the original on 11 March 2021.
- ↑ Tan, Lili (15 March 2021). "'Hunger Games of Vaccines': People Jump the Line to Get Vaccine Prior to Eligibility". National Broadcasting Company. Archived from the original on 16 March 2021.
- ↑ O'Neill, Eilis (9 February 2021). "How People Are Jumping the COVID-19 Vaccine Line". National Public Radio. Archived from the original on 15 March 2021.
- ↑ Rosenthal, Elisabeth (2 February 2021). "With Demand Far Exceeding Supply, It Matters That People Are Jumping the Vaccine Line". Kaiser Family Foundation. Archived from the original on 2 February 2021.