
Alamethicin is a channel-forming peptide antibiotic, produced by the fungus Trichoderma viride. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib. This residue strongly induces formation of alpha-helical structure. The peptide sequence is:

Aminolevulinic acid synthase (ALA synthase, ALAS, or delta-aminolevulinic acid synthase) is an enzyme (EC 2.3.1.37) that catalyzes the synthesis of δ-aminolevulinic acid (ALA) the first common precursor in the biosynthesis of all tetrapyrroles such as hemes, cobalamins and chlorophylls. The reaction is as follows:
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined together to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Novobiocin, also known as albamycin or cathomycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the order Actinobacteria. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.
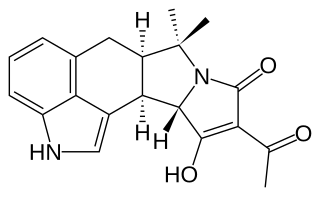
Cyclopiazonic acid (α-CPA), a mycotoxin and a fungal neurotoxin, is made by the molds Aspergillus and Penicillium.. It is an indole-tetramic acid that serves as a toxin due to its ability to inhibit calcium-dependent ATPases found in the endoplasmic and sarcoplasmic reticulum. This inhibition disrupts the muscle contraction-relaxation cycle and the calcium gradient that is maintained for proper cellular activity in cells.

Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Bacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can only synthesize 11 of the 20 standard amino acids, and in time of accelerated growth, histidine can be considered an essential amino acid.

Tryptophan hydroxylase (TPH) is an enzyme (EC 1.14.16.4) involved in the synthesis of the neurotransmitter serotonin. Tyrosine hydroxylase, phenylalanine hydroxylase, and tryptophan hydroxylase together constitute the family of biopterin-dependent aromatic amino acid hydroxylases. TPH catalyzes the following chemical reaction

Colitose is a mannose-derived 3,6-dideoxysugar produced by certain bacteria. It is a constituent of the lipopolysaccharide.

Enterobactin is a high affinity siderophore that acquires iron for microbial systems. It is primarily found in Gram-negative bacteria, such as Escherichia coli and Salmonella typhimurium.

Thiolases, also known as acetyl-coenzyme A acetyltransferases (ACAT), are enzymes which convert two units of acetyl-CoA to acetoacetyl CoA in the mevalonate pathway.

Orotate phosphoribosyltransferase (OPRTase) or orotic acid phosphoribosyltransferase is an enzyme involved in pyrimidine biosynthesis. It catalyzes the formation of orotidine 5'-monophosphate (OMP) from orotate and phosphoribosyl pyrophosphate. In yeast and bacteria, orotate phosphoribosyltransferase is an independent enzyme with a unique gene coding for the protein, whereas in mammals and other multicellular organisms, the catalytic function is carried out by a domain of the bifunctional enzyme UMP synthase (UMPS).
In enzymology, a 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase (EC 1.3.1.28) is an enzyme that catalyzes the chemical reaction

Morpheeins are proteins that can form two or more different homo-oligomers, but must come apart and change shape to convert between forms. The alternate shape may reassemble to a different oligomer. The shape of the subunit dictates which oligomer is formed. Each oligomer has a finite number of subunits (stoichiometry). Morpheeins can interconvert between forms under physiological conditions and can exist as an equilibrium of different oligomers. These oligomers are physiologically relevant and are not misfolded protein; this distinguishes morpheeins from prions and amyloid. The different oligomers have distinct functionality. Interconversion of morpheein forms can be a structural basis for allosteric regulation. A mutation that shifts the normal equilibrium of morpheein forms can serve as the basis for a conformational disease. Features of morpheeins can be exploited for drug discovery. The dice image represents a morpheein equilibrium containing two different monomeric shapes that dictate assembly to a tetramer or a pentamer. The one protein that is established to function as a morpheein is porphobilinogen synthase, though there are suggestions throughout the literature that other proteins may function as morpheeins.

Isochorismate Pyruvate Lyase (IPL) is an enzyme responsible for catalyzing part of the pathway involved in the formation of salicylic acid. More specifically, IPL will use isochorismate as a substrate and convert it into salicylate and pyruvate. IPL is a PchB enzyme originating from the pchB gene in Pseudomonas aeruginosa.

Bacillibactin is a catechol-based siderophore secreted by members of the genus Bacillus, including Bacillus anthracis and Bacillus subtilis. It is involved in the chelation of ferric iron (Fe3+) from the surrounding environment and is subsequently transferred into the bacterial cytoplasm via the use of ABC transporters.

Fumiquinazolines are bio-active isolates of Aspergillus.
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions.

Mirubactin is a siderophore produced by the bacterium Actinosynnema mirum.A.mirum was first isolated from the Raritan River in New Jersey in 1976, and its full genome sequence was published in 2009. In 2012, mirubactin was isolated and characterized, and the biosynthesis was connected with the gene cluster Amir_2714-Amir_2728, since renamed mrbA-mrbO.

















