
Gap junctions are specialized intercellular connections between a multitude of animal cell-types. They directly connect the cytoplasm of two cells, which allows various molecules, ions and electrical impulses to directly pass through a regulated gate between cells.

Adenoviruses are medium-sized, nonenveloped viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from their initial isolation from human adenoids in 1953.

Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.

In biology, a connexon, also known as a connexin hemichannel, is an assembly of six proteins called connexins that form the pore for a gap junction between the cytoplasm of two adjacent cells. This channel allows for bidirectional flow of ions and signaling molecules. The connexon is the hemichannel supplied by a cell on one side of the junction; two connexons from opposing cells normally come together to form the complete intercellular gap junction channel. In some cells, the hemichannel itself is active as a conduit between the cytoplasm and the extracellular space, allowing the transference of ions and small molecules lower than 1-2 KDa. Little is known about this function of connexons besides the new evidence suggesting their key role in intracellular signaling. In still other cells connexons have been shown to occur in mitochondrial membranes and appear to play a role in heart ischaemia.

Connexins (Cx), or gap junction proteins, are structurally related transmembrane proteins that assemble to form vertebrate gap junctions. An entirely different family of proteins, the innexins, form gap junctions in invertebrates. Each gap junction is composed of two hemichannels, or connexons, which consist of homo- or heterohexameric arrays of connexins, and the connexon in one plasma membrane docks end-to-end with a connexon in the membrane of a closely opposed cell. The hemichannel is made of six connexin subunits, each of which consist of four transmembrane segments. Gap junctions are essential for many physiological processes, such as the coordinated depolarization of cardiac muscle, proper embryonic development, and the conducted response in microvasculature. Connexins also have non-channel dependant functions relating to cytoskeleton and cell migration. For these reasons, mutations in connexin-encoding genes can lead to functional and developmental abnormalities.

A polydnavirus (PDV) or more recently, polydnaviriform is a member of the family Polydnaviridae of insect viruses. There are two genera in the family: Bracovirus and Ichnovirus. Polydnaviruses form a symbiotic relationship with parasitoid wasps;. The larvae of wasps in both of those groups are themselves parasitic on Lepidoptera, and the polydnaviruses are important in circumventing the immune response of their parasitized hosts. Little or no sequence homology exists between BV and IV, suggesting that the two genera have been evolving independently for a long time.
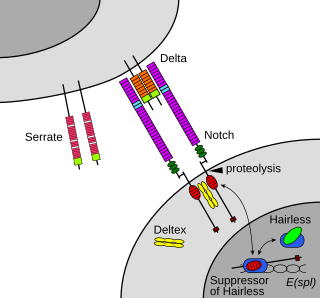
In biology, juxtacrine signalling is a type of cell–cell or cell–extracellular matrix signalling in multicellular organisms that requires close contact. In this type of signalling, a ligand on one surface binds to a receptor on another adjacent surface. Hence, this stands in contrast to releasing a signaling molecule by diffusion into extracellular space, the use of long-range conduits like membrane nanotubes and cytonemes or the use of extracellular vesicles like exosomes or microvesicles. There are three types of juxtacrine signaling:
- A membrane ligand and a membrane protein of two adjacent cells interact.
- A communicating junction links the intracellular compartments of two adjacent cells, allowing transit of relatively small molecules.
- An extracellular matrix glycoprotein and a membrane protein interact.

Pannexins are a family of vertebrate proteins identified by their homology to the invertebrate innexins. While innexins are responsible for forming gap junctions in invertebrates, the pannexins have been shown to predominantly exist as large transmembrane channels connecting the intracellular and extracellular space, allowing the passage of ions and small molecules between these compartments.
Innexins are transmembrane proteins that form gap junctions in invertebrates. Gap junctions are composed of membrane proteins that form a channel permeable to ions and small molecules connecting the cytoplasm of adjacent cells. Although gap junctions provide similar functions in all multicellular organisms, it was not known what proteins invertebrates used for this purpose until the late 1990s. While the connexin family of gap junction proteins was well-characterized in vertebrates, no homologues were found in non-chordates.
Membrane channels are a family of biological membrane proteins which allow the passive movement of ions, water (aquaporins) or other solutes to passively pass through the membrane down their electrochemical gradient. They are studied using a range of channelomics experimental and mathematical techniques. Insights have suggested endocannabinoids (eCBs) as molecules that can regulate the opening of these channels during diverse conditions.

Gap junction alpha-1 protein (GJA1), also known as connexin 43 (Cx43), is a protein that in humans is encoded by the GJA1 gene on chromosome 6. As a connexin, GJA1 is a component of gap junctions, which allow for gap junction intercellular communication (GJIC) between cells to regulate cell death, proliferation, and differentiation. As a result of its function, GJA1 is implicated in many biological processes, including muscle contraction, embryonic development, inflammation, and spermatogenesis, as well as diseases, including oculodentodigital dysplasia (ODDD), heart malformations, and cancers.

Gap junction beta-1 protein (GJB1), also known as connexin 32 (Cx32) is a transmembrane protein that in humans is encoded by the GJB1 gene. Gap junction beta-1 protein is a member of the gap junction connexin family of proteins that regulates and controls the transfer of communication signals across cell membranes, primarily in the liver and peripheral nervous system.

Coxsackievirus and adenovirus receptor (CAR) is a protein that in humans is encoded by the CXADR gene. The protein encoded by this gene is a type I membrane receptor for group B coxsackie viruses and subgroup C adenoviruses. CAR protein is expressed in several tissues, including heart, brain, and, more generally, epithelial and endothelial cells. In cardiac muscle, CAR is localized to intercalated disc structures, which electrically and mechanically couple adjacent cardiomyocytes. CAR plays an important role in the pathogenesis of myocarditis, dilated cardiomyopathy, and in arrhythmia susceptibility following myocardial infarction or myocardial ischemia. In addition, an isoform of CAR (CAR-SIV) has been recently identified in the cytoplasm of pancreatic beta cells. It's been suggested that CAR-SIV resides in the insulin secreting granules and might be involved in the virus infection of these cells.

Gap junction gamma-1 protein (GJC1), also known as gap junction alpha-7 protein (GJA7) and connexin 45 (Cx45) — is a protein that in humans is encoded by the GJC1 gene.

Gap junction delta-4 protein (GJD4), also known as connexin-40.1 (Cx40.1), is a protein that in humans is encoded by the GJD4 gene.

Gap junction delta-2 (GJD2), also known as connexin-36 (Cx36) or gap junction alpha-9 (GJA9), is a protein that in humans is encoded by the GJD2 gene.

Gap junction beta-7 protein (GJB7), also known as connexin-25 (Cx25), is a protein that in humans is encoded by the GJB7 gene.

Gap junction alpha-10 protein, also known as connexin-62 (Cx62), is a protein that in humans is encoded by the GJA10 gene.

Pannexin 1 is a protein in humans that is encoded by the PANX1 gene.
"Intercellular communication" refers to the varying ways and structures biological cells use to communicate with each other directly or through their environment. Not all cells use all of the proteins or mechanisms and there are likely to be more yet to be discovered. Components of each type of intercellular communication may be involved in more than one type of communication making attempts at clearly separating the types of communication listed somewhat futile. The sections are loosely compiled from various areas of research rather than by a systematic attempt of classification by functional or structural characteristics.


















