GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved G domain common to many GTPases.

Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-dependent pulling action in muscle contraction and pseudopod advancement. Microfilaments have a tough, flexible framework which helps the cell in movement.

Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42-kDa, with a diameter of 4 to 7 nm.

The Wiskott–Aldrich Syndrome protein (WASp) is a 502-amino acid protein expressed in cells of the hematopoietic system that in humans is encoded by the WAS gene. In the inactive state, WASp exists in an autoinhibited conformation with sequences near its C-terminus binding to a region near its N-terminus. Its activation is dependent upon CDC42 and PIP2 acting to disrupt this interaction, causing the WASp protein to 'open'. This exposes a domain near the WASp C-terminus that binds to and activates the Arp2/3 complex. Activated Arp2/3 nucleates new F-actin.

Podosomes are conical, actin-rich structures found on the outer surface of the plasma membrane of animal cells. Their size ranges from approximately 0.5 µm to 2.0 µm in diameter. While usually situated on the periphery of the cellular membrane, these unique structures display a polarized pattern of distribution in migrating cells, situating at the front border between the lamellipodium and lamellum. Their primary purpose is connected to cellular motility and invasion; therefore, they serve as both sites of attachment and degradation along the extracellular matrix. Many different specialized cells exhibit these dynamic structures such as invasive cancer cells, osteoclasts, vascular smooth muscle cells, endothelial cells, and certain immune cells like macrophages and dendritic cells.
The lamellipodium is a cytoskeletal protein actin projection on the leading edge of the cell. It contains a quasi-two-dimensional actin mesh; the whole structure propels the cell across a substrate. Within the lamellipodia are ribs of actin called microspikes, which, when they spread beyond the lamellipodium frontier, are called filopodia. The lamellipodium is born of actin nucleation in the plasma membrane of the cell and is the primary area of actin incorporation or microfilament formation of the cell.

Cortactin is a monomeric protein located in the cytoplasm of cells that can be activated by external stimuli to promote polymerization and rearrangement of the actin cytoskeleton, especially the actin cortex around the cellular periphery. It is present in all cell types. When activated, it will recruit Arp2/3 complex proteins to existing actin microfilaments, facilitating and stabilizing nucleation sites for actin branching. Cortactin is important in promoting lamellipodia formation, invadopodia formation, cell migration, and endocytosis.
The Rho family of GTPases is a family of small signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions.

Actin-related protein 3 is a protein that in humans is encoded by the ACTR3 gene.

Abl interactor 1 also known as Abelson interactor 1 (Abi-1) is a protein that in humans is encoded by the ABI1 gene.

Wiskott-Aldrich syndrome protein family member 2 is a protein that in humans is encoded by the WASF2 gene.
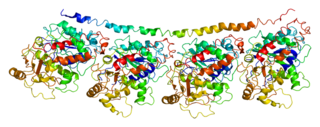
Tubulin alpha-4A chain is a protein that in humans is encoded by the TUBA4A gene.

Neural Wiskott-Aldrich syndrome protein is a protein that in humans is encoded by the WASL gene.

Wiskott–Aldrich syndrome protein family member 1, also known as WASP-family verprolin homologous protein 1 (WAVE1), is a protein that in humans is encoded by the WASF1 gene.

Nck-associated protein 1 is a protein that in humans is encoded by the NCKAP1 gene.

Neurabin-2 is a protein that in humans is encoded by the PPP1R9B gene.

Cytoplasmic FMR1-interacting protein 1 is a protein that in humans is encoded by the CYFIP1 gene.

Protein cordon-bleu is a protein that in humans is encoded by the COBL gene.

The Actin assembly-inducing protein (ActA) is a protein encoded and used by Listeria monocytogenes to propel itself through a mammalian host cell. ActA is a bacterial surface protein comprising a membrane-spanning region. In a mammalian cell the bacterial ActA interacts with the Arp2/3 complex and actin monomers to induce actin polymerization on the bacterial surface generating an actin comet tail. The gene encoding ActA is named actA or prtB.

Arp2/3 complex is a seven-subunit protein complex that plays a major role in the regulation of the actin cytoskeleton. It is a major component of the actin cytoskeleton and is found in most actin cytoskeleton-containing eukaryotic cells. Two of its subunits, the Actin-Related Proteins ARP2 and ARP3, closely resemble the structure of monomeric actin and serve as nucleation sites for new actin filaments. The complex binds to the sides of existing ("mother") filaments and initiates growth of a new ("daughter") filament at a distinctive 70 degree angle from the mother. Branched actin networks are created as a result of this nucleation of new filaments. The regulation of rearrangements of the actin cytoskeleton is important for processes like cell locomotion, phagocytosis, and intracellular motility of lipid vesicles.

















