
Paracrine signaling is a form of cell signaling, a type of cellular communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour of those cells. Signaling molecules known as paracrine factors diffuse over a relatively short distance, as opposed to cell signaling by endocrine factors, hormones which travel considerably longer distances via the circulatory system; juxtacrine interactions; and autocrine signaling. Cells that produce paracrine factors secrete them into the immediate extracellular environment. Factors then travel to nearby cells in which the gradient of factor received determines the outcome. However, the exact distance that paracrine factors can travel is not certain.
The Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt is a portmanteau created from the names Wingless and Int-1. Wnt signaling pathways use either nearby cell-cell communication (paracrine) or same-cell communication (autocrine). They are highly evolutionarily conserved in animals, which means they are similar across animal species from fruit flies to humans.

A morphogen is a substance whose non-uniform distribution governs the pattern of tissue development in the process of morphogenesis or pattern formation, one of the core processes of developmental biology, establishing positions of the various specialized cell types within a tissue. More specifically, a morphogen is a signaling molecule that acts directly on cells to produce specific cellular responses depending on its local concentration.
The Hedgehog signaling pathway is a signaling pathway that transmits information to embryonic cells required for proper cell differentiation. Different parts of the embryo have different concentrations of hedgehog signaling proteins. The pathway also has roles in the adult. Diseases associated with the malfunction of this pathway include cancer.
Frzb is a Wnt-binding protein especially important in embryonic development. It is a competitor for the cell-surface G-protein receptor Frizzled.
Catenin beta-1, also known as beta-catenin (β-catenin), is a protein that in humans is encoded by the CTNNB1 gene.

Frizzled is a family of atypical G protein-coupled receptors that serve as receptors in the Wnt signaling pathway and other signaling pathways. When activated, Frizzled leads to activation of Dishevelled in the cytosol.

Alpha-catenin functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and alpha-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when alpha-catenin is not in a molecular complex with beta-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. Alpha catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, beta-catenin and alpha-catenin has not been isolated.

Axin-1 is a protein that in humans is encoded by the AXIN1 gene.

Proto-oncogene Wnt-1, or Proto-oncogene Int-1 homolog is a protein that in humans is encoded by the WNT1 gene.

Frizzled-5(Fz-5) is a protein that in humans is encoded by the FZD5 gene.
Frizzled-8(Fz-8) is a protein that in humans is encoded by the FZD8 gene.

Ras GTPase-activating-like protein IQGAP1 (IQGAP1) also known as p195 is a ubiquitously expressed protein that in humans is encoded by the IQGAP1 gene. IQGAP1 is a scaffold protein involved in regulating various cellular processes ranging from organization of the actin cytoskeleton, transcription, and cellular adhesion to regulating the cell cycle.

Segment polarity protein dishevelled homolog DVL-1 is a protein that in humans is encoded by the DVL1 gene.

Secreted frizzled-related protein 1, also known as SFRP1, is a protein which in humans is encoded by the SFRP1 gene.

Low-density lipoprotein receptor-related protein 6 is a protein that in humans is encoded by the LRP6 gene. LRP6 is a key component of the LRP5/LRP6/Frizzled co-receptor group that is involved in canonical Wnt pathway.

Wnt inhibitory factor 1 is a protein that in humans is encoded by the WIF1 gene. WIF1 is a lipid-binding protein that binds to Wnt proteins and prevents them from triggering signalling.

Wingless-type MMTV integration site family, member 2, also known as WNT2, is a human gene.
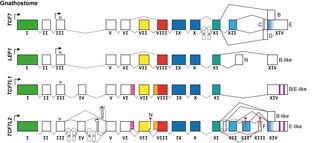
The TCF/LEF family is a group of genes that encode transcription factors which bind to DNA through a SOX-like high mobility group domain. They are involved in the Wnt signaling pathway, particularly during embryonic and stem-cell development, but also had been found to play a role in cancer and diabetes. TCF/LEF factors recruit the coactivator beta-catenin to enhancer elements of genes they target. They can also recruit members of the Groucho family of corepressors.
Naked cuticle (Nkd) is a conserved family of intracellular proteins encoded in most animal genomes. The original mutants were discovered by 1995 Nobel laureates Christiane Nüsslein-Volhard and Eric F. Wieschaus and colleagues in their genetic screens for pattern-formation mutants in the fruit fly Drosophila melanogaster. The Nkd gene family was first cloned in the laboratory of Matthew P. Scott. Like many cleverly named fly mutants, the name "naked cuticle" derives from the fact that mutants lack most of the hair-like protrusions from their ventral cuticle and thus appear "naked".














