Strychnine is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eyes or mouth, causes poisoning which results in muscular convulsions and eventually death through asphyxia. While it is no longer used medicinally, it was used historically in small doses to strengthen muscle contractions, such as a heart and bowel stimulant and performance enhancing drug. The most common source is from the seeds of the Strychnos nux-vomica tree.

The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams.

An oxime is a chemical compound belonging to the imines, with the general formula RR'C=NOH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

The benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals. The reaction produces an acyloin. In the classic application benzaldehyde is converted to benzoin.
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.

Wilhelm Rudolph Fittig was a German chemist. Fittig discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. He studied the action of sodium on ketones and hydrocarbons. He discovered the Fittig reaction or Wurtz–Fittig reaction for the synthesis of alkylbenzenes, he proposed a diketone structure for benzoquinone and isolated phenanthrene from coal tar. He discovered and synthesized the first lactones and investigated structures of piperine naphthalene and fluorene.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.
The Lossen rearrangement is the conversion of a hydroxamate ester to an isocyanate. Typically O-acyl, sulfonyl, or phosphoryl O-derivative are employed. The isocyanate can be used further to generate ureas in the presence of amines or generate amines in the presence of H2O.
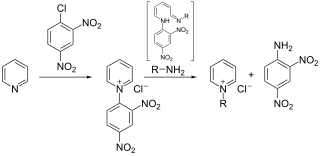
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke.

Zincke aldehydes, or 5-aminopenta-2,4-dienals, are the product of the reaction of a pyridinium salt with two equivalents of any secondary amine, followed by basic hydrolysis. Using secondary amines the Zincke reaction takes on a different shape forming Zincke aldehydes in which the pyridine ring is ring-opened with the terminal iminium group hydrolyzed to an aldehyde. The use of the dinitrophenyl group for pyridine activation was first reported by Theodor Zincke. The use of cyanogen bromide for pyridine activation was independently reported by W. König:
Walter Julius Reppe was a German chemist. He is notable for his contributions to the chemistry of acetylene.

The Diels–Reese Reaction is a reaction between hydrazobenzene and dimethyl acetylenedicarboxylate first reported in 1934 by Otto Diels and Johannes Reese. Later work by others extended the reaction scope to include substituted hydrazobenzenes. The exact mechanism is not known. By changing the acidic or basic nature of the solvent, the reaction gives different products. With acetic acid as solvent (acidic), the reaction gives an diphenylpyrazolone. With xylene as solvent (neutral), the reaction gives an indole. With pyridine as solvent (basic), the reaction gives a carbomethoxyquinoline which can be degraded to a dihydroquinoline.

Adolph Strecker was a German chemist who is remembered primarily for his work with amino acids.

Thiocyanogen, (SCN)2, is a pseudohalogen derived from the pseudohalide thiocyanate, [SCN]−. This hexatomic compound exhibits C2 point group symmetry and has the connectivity NCS-SCN. The oxidation ability is greater than bromine. It reacts with water:

The Neber rearrangement is an organic reaction in which a ketoxime is converted into an alpha-aminoketone via a rearrangement reaction.

Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers. Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a phenyl substituent; the parent member of the phenylacetaldehyde class of compounds. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes.

The haloform reaction is a chemical reaction where a haloform (CHX3, where X is a halogen) is produced by the exhaustive halogenation of a methyl ketone (RCOCH3, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform (CHCl3), bromoform (CHBr3), or iodoform (CHI3). [Note:fluoroform (CHF3) can't be prepared in this way.]

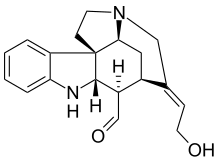
Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field.

Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.
Radical theory is an obsolete scientific theory in chemistry describing the structure of organic compounds. The theory was pioneered by Justus von Liebig, Friedrich Wöhler and Auguste Laurent around 1830 and is not related to the modern understanding of free radicals. In this theory, organic compounds were thought to exist as combinations of radicals that could be exchanged in chemical reactions just as chemical elements could be interchanged in inorganic compounds.